
Interpretation: Given the decomposition of
Concept Introduction:
The integrated rate law equation explains how the concentrations of reactants change with time.
Consider a first order
The concentration of the reactant A at time t is given by the below equation
Where,
The integrated rate law for this first order reaction is obtained by taking the natural logarithm of both sides of
That is,
Using Dalton's law, the partial pressure of formic acid is given by
Where,
The order of the reaction can be determined from a plot of concentration against time.
If we plot concentration against time, and if the curve is linear, the reaction is a zero order reaction.
If we plot log of concentration against time and if the curve is linear, the reaction is a first order reaction.
If we plot concentration inverse against time and if the curve is linear, the reaction is a second order reaction.
Answer to Problem 11.50PAE
Solution: The rate constant is
Explanation of Solution
Given Information: The table containing the total pressures in the reaction vessel during the decomposition of
Decomposition of
The initial partial pressure of
Calculate the partial pressure of
Partial pressure of
Partial pressure of
Partial pressure of
Therefore, the total pressure at any given time t is given as
| Time(t) | Total pressure |
|
| 0 | 491.7 | 491.7 |
| 185.3 | 549.6 | 434.0 |
| 242.8 | 566.6 | 417.0 |
| 304.5 | 584.1 | 399.5 |
| 362.7 | 599.9 | 383.7 |
| 429.5 | 617.2 | 366.4 |
| 509.7 | 637.0 | 346.6 |
| 606.3 | 659.5 | 324.1 |
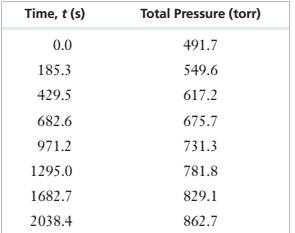
We need to plot these values of partial pressure with time to see if the reaction is zero order or not
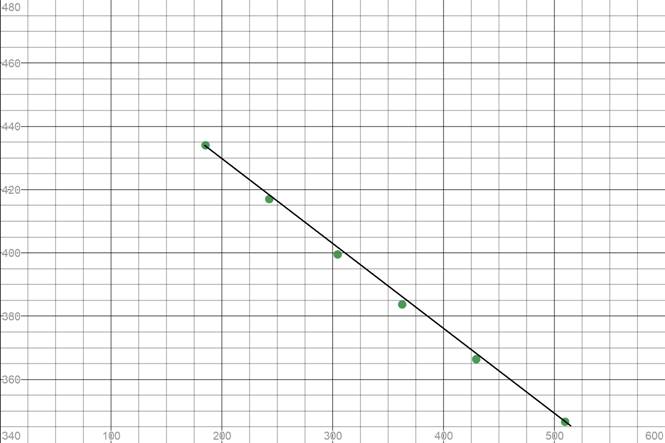
As some points do not lie on the straight line, the curve is not linear. Thus, it is not a zero order reaction.
| Time(t) | |
|
| 0 | 491.7 | 6.1978687744 |
| 185.3 | 434.0 | 6.0730445341 |
| 242.8 | 417.0 | 6.0330862218 |
| 304.5 | 399.5 | 5.9902137652 |
| 362.7 | 383.7 | 5.9498609973 |
| 429.5 | 366.4 | 5.9037256328 |
| 509.7 | 346.6 | 5.8481713773 |
| 606.3 | 324.1 | 5.7810521101 |
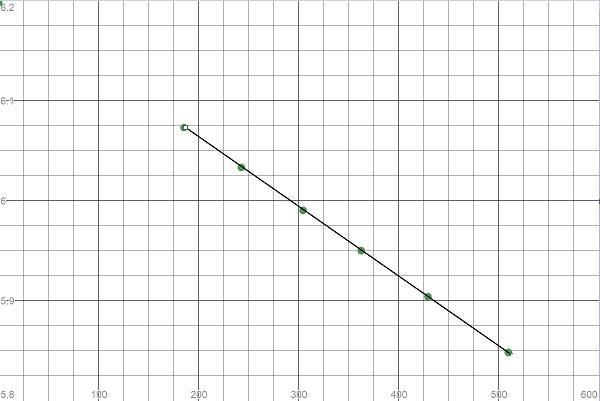
Here we see the curve is linear and thus the reaction is a first order reaction.
To calculate the rate constant, we need the negative slope of the line in the plot
Hence, the rate constant is the negative of the slope obtained. It is equal to
The concept of integrated rate law and the manipulation the data into a plot helps in determining the order of the decomposition of
Want to see more full solutions like this?
Chapter 11 Solutions
Bundle: Chemistry for Engineering Students, Loose-Leaf Version, 4th + OWLv2 with MindTap Reader with Student Solutions Manual, 1 term (6 months) Printed Access Card
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning  ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





