
The reaction
NO(g) + O,(g) — NO,(g) + 0(g)
plays a role in the formation of nitrogen dioxide in automobile engines. Suppose that a series of experiments measured the
| [NO] |
(mol L ’)
(mol L 1)
Derive a rate law for the reaction and determine the value of the rate constant.
Interpretation: Given the experimental data obtained for a reaction at 500K, derive the rate law for the reaction and find the value of the rate constant.
Concept Introduction: Orders of reaction are constantly determined by doing experiments. Consequently without experimental information, we can't conclude anything about the order of a reaction just by having a look at the equation for the reaction. By doing experiments involving a reaction between A and B, the rate of the reaction is identified to be related to the concentrations of A and B as follows:
This is the Rate Equation.
Where,
Rate is in the units of mol dm-3s-1
k is the rate constant
A, B- concentrations in mol dm-3
a - Order of reaction with respect to A
b- Order of reaction with respect to B
If temperature is given, the rate is usually considered to be a function of the initial concentrations of the reactants A and B.
Answer to Problem 11.36PAE
Solution: The rate law of the reaction is
Explanation of Solution
Given information: Reaction:
Experimental Data
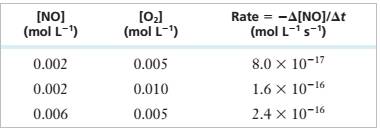
Step 1: For the reaction:
The rate law can be determined using the rate equation as follows:
Where,
a= Order of the reaction with respect to NO
b= Order of the reaction with respect to
Step 2: From the first, second and third rows of the given experimental data,
Step 3: Divide (2) by (1), we get
Step 4: Divide (3) by (1), we get
Step 5: Rate Equation = >
Step 6: Substitute a=1, b=1 values in (1)
It does not make a difference what the number of reactants there are. The concentration of every reactant will be present in the rate equation, raised to some power. These powers resemble the individual orders with respect to each reactant. The sum of these powers results in the overall order of the reaction. The rate constant will be a constant value for a given reaction only if the concentration of the reactants is changed without changing any other factors.
Want to see more full solutions like this?
Chapter 11 Solutions
Bundle: Chemistry for Engineering Students, Loose-Leaf Version, 4th + OWLv2 with MindTap Reader with Student Solutions Manual, 1 term (6 months) Printed Access Card
- Q5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forwardCalculate the proton and carbon chemical shifts for this structurearrow_forwardA. B. b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion and B. is a neutral molecule. One of these molecules is a highly reactive compound first characterized in frozen noble gas matrices, that self-reacts rapidly at temperatures above liquid nitrogen temperature. The other compound was isolated at room temperature in the early 1960s, and is a stable ligand used in organometallic chemistry. Which molecule is the more stable molecule, and why?arrow_forward
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





