
Experimental data are listed here for the reaction ![Chapter 11, Problem 11.20PAE, Experimental data are listed here for the reaction B: Time (s) IB] (mol/L) 0.00 0.000 10.0 0.326](https://content.bartleby.com/tbms-images/9781337398909/Chapter-11/images/html_98909-11-11.20pae_image001.jpg) B:
B:
| Time (s) | IB] (mol/L) |
| 0.00 | 0.000 |
| 10.0 | 0.326 |
| 20.0 | 0.572 |
| 30.0 | 0.750 |
| 40.0 | 0.890 |
- Prepare a graph from these data, connect the points with a smooth line, and calculate the rate of change of [B] for each 10-s interval from 0.0 to 40.0 s. Does the rate of change decrease from one time interval to the next? Suggest a reason for this result.
- How is the rate of change of [AJ related to the rate of change of [B] in each time interval? Calculate the rate of change of [AJ for the time interval from 10.0 to 20.0 s.
- What is the instantaneous rate, A[B]/Ar, when [BI = 0.750 mol/L?
a)
Interpretation:
A graph based on the given data must be plotted and the rate of change of [B] for every 10 s interval must be calculated.
Concept Introduction:
- Chemical reactions proceed at a certain rate which is represented in terms of the change in concentration over a certain period of time
- The rate can be expressed either in terms of a decrease in concentration of the reactants or an increase in the concentration of products.
Answer to Problem 11.20PAE
Solution:
The plot is depicted below.
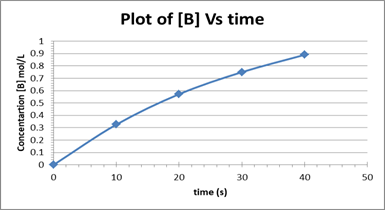
The rate of change of [B] decreases from one-time interval to the next.
Explanation of Solution
The given reaction is:
A plot of concentration of [B] vs time based on the given data is shown below:
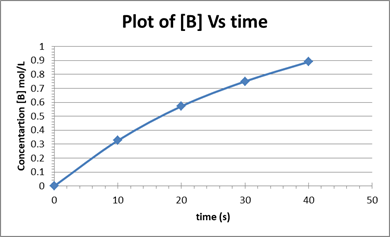
As per the above calculations, the rate of change decreases from one-time interval to the next. This is because as time increases the concentration of reactants decreases as a result the rate of formation of the products will also decrease.
(b)
Interpretation:
The relation between the rate of change of [A] and the rate of change of [B] must be explained. The rate of change of [A] for the time interval from 10.0 to 20.0 s should be calculated.
Concept Introduction:
- Chemical reactions proceed at a certain rate which is represented in terms of the change in concentration over a certain period of time
- The rate can be expressed either in terms of a decrease in concentration of the reactants or an increase in the concentration of products.
Answer to Problem 11.20PAE
Solution:
The rate of change of A is half that of B and for time interval 10 to 20 s rate of change of [A] is
Explanation of Solution
The given reaction is:
Based on the stoichiometry of this reaction, the rate can be expressed as:
For the time interval between t = 10 to t = 20 s:
(c)
Interpretation:
The instantaneous rate must be calculated when [B] = 0.750 mol/L
Concept Introduction:
- Chemical reactions proceed at a certain rate which is represented in terms of the change in concentration over a certain period of time
- The rate can be expressed either in terms of a decrease in concentration of the reactants or an increase in the concentration of products.
- Average rate of a reaction can be defined as the difference in the concentrations measured at two different times whereas, instantaneous rate can be defined as the rate of a reaction at a particular instant in time.
Answer to Problem 11.20PAE
Solution: Rate = 0.200 mol/L-s
Explanation of Solution
Instantaneous rate can be deduced by drawing a tangent at the point of the curve that corresponds to a particular instant. The slope of the tangent gives the instantaneous rate. In this case the value of [B] = 0.075 mol/L corresponds to a time t = 30 sec. The tangent and the slope are depicted in the plot shown below:
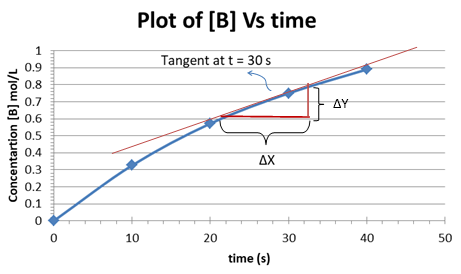
Therefore, the instantaneous rate is
Want to see more full solutions like this?
Chapter 11 Solutions
Bundle: Chemistry for Engineering Students, Loose-Leaf Version, 4th + OWLv2 with MindTap Reader with Student Solutions Manual, 1 term (6 months) Printed Access Card
- What is the IUPAC name of the following compound? CH₂CH₂ H CI H₂CH₂C H CH₂ Selected Answer: O (35,4R)-4 chloro-3-ethylpentane Correctarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forward
- Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardGiven 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





