
(a)
Interpretation:
Given compound has to be named using
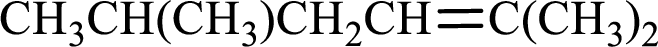
Concept Introduction:
A common nomenclature of naming organic compounds has been developed by IUPAC. By usage of this nomenclature or rules, memorizing of names of organic compounds is not necessary.
IUPAC rules for naming
There are about five rules that has to be followed for naming an alkene and an
- The longest continuous carbon chain in the compound that contains double bond or triple has to be identified. This is known as parent compound.
- Suffix “–ane” (in name of
alkane ) is replaced with “-ene” for alkene or “-yne” for alkyne. - Numbering has to be done so that the lowest number is given to the double or triple bond.
- Naming and numbering has to be given for each atom or group that is attached to the parent chain. Numbering has to be done in a way that substituents get the least numbering.
- If the alkenes have more than one double bond they are called as alkadienes (two double bonds) or alkatrienes (three double bonds). Appropriate suffix has to be used depending on the number of multiple bonds present in the compound.
(a)
Explanation of Solution
Given compound is,
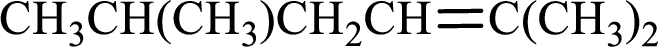
Longest carbon chain with double bond is found to contain five carbon atoms. Therefore, the parent alkane is hexane. As a double bond is present, the alkene name is hexene.
Numbering has to be given in a way that the double bond gets the least numbering. In this case, double bond is present between carbon-2 and carbon-3. Therefore, the parent alkene is 2-hexene.
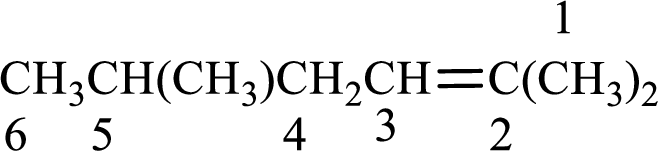
The substituent present in the given structure are a methyl group on carbon-5 and a methyl group on carbon-2. Number has to be added before the substituent names which indicate the carbon atom in which it is present. This gives the name of alkene as 2,5-dimethyl-2-hexene.
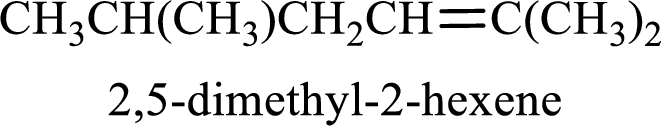
Longest carbon chain containing double bond is hexane. Position of double bond is 2-hexene. Substituents present in the chain are 2,5-dimethyl. IUPAC name of the alkene given is 2,5-dimethyl-2-hexene.
(b)
Interpretation:
Given compound has to be named using IUPAC nomenclature.

Concept Introduction:
Refer part (a).
(b)
Explanation of Solution
Given compound is,

Longest carbon chain with triple bond is found to contain four carbon atoms. Therefore, the parent alkane is butane. As a triple bond is present, the alkyne name is butyne.
Numbering has to be given in a way that the triple bond gets the least numbering. In this case, triple bond is present between carbon-1 and carbon-2. Therefore, the parent alkyne is 1-butyne.
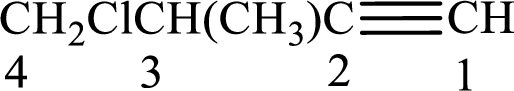
The substituents present in the given structure are a methyl group on carbon-3 and a chlorine atom on carbon-4. Substituents have to be arranged in alphabetical order. Number has to be added before the substituent names which indicate the carbon atom in which it is present. This gives the name of alkyne as 4-chloro-3-methyl-1-butyne.
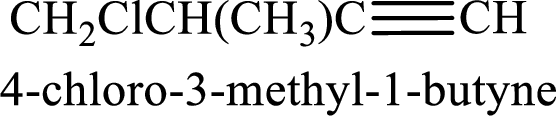
Longest carbon chain containing triple bond is butane. Position of triple bond is 1-butyne. Substituents present in the chain are 4-chloro-3-methyl. IUPAC name of the alkyne given is 4-chloro-3-methyl-1-butyne.
(c)
Interpretation:
Given compound has to be named using IUPAC nomenclature.
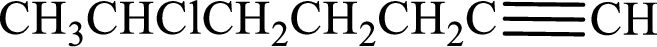
Concept Introduction:
Refer part (a).
(c)
Explanation of Solution
Given compound is,
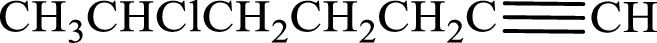
Longest carbon chain with triple bond is found to contain seven carbon atoms. Therefore, the parent alkane is heptane. As a triple bond is present, the alkyne name is heptyne.
Numbering has to be given in a way that the double bond gets the least numbering. In this case, double bond is present between carbon-1 and carbon-2. Therefore, the parent alkene is 1-heptyne.
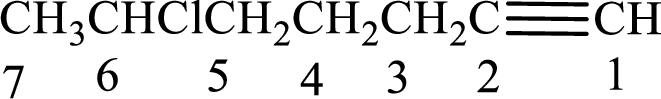
The substituent present in the given structure is a chlorine atom on carbon-6. Number has to be added before the substituent names which indicate the carbon atom in which it is present. This gives the name of alkyne as 6-chloro-1-heptyne.
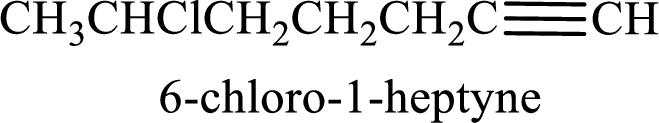
Longest carbon chain containing double bond is heptane. Position of triple bond is 1-heptyne. Substituents present in the chain are 6-chloro. IUPAC name of the alkyne given is 6-chloro-1-heptyne.
(d)
Interpretation:
Given compound has to be named using IUPAC nomenclature.
Concept Introduction:
A common nomenclature of naming organic compounds has been developed by IUPAC. By usage of this nomenclature or rules, memorizing of names of organic compounds is not necessary.
IUPAC rules for naming cycloalkenes:
- The number of carbon atoms present in the ring is counted and the name of the alkane that has the same number of carbon atoms is given by adding prefix “cyclo-” to the alkane name. Suffix “-ane” is changed as “-ene”.
- The double bond that is present in the ring is given always the number 1.
- If the ring is substituted, then the names of the group or atoms have to be placed before the name of cycloalkene.
- If the ring contains more than one substituent, then the numbers has to be used in a way that it gives the lowest position for the substituents following position 1 for the double bond.
(d)
Explanation of Solution
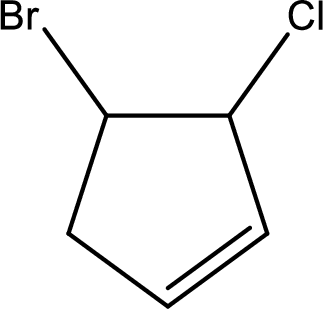
Given compound is,
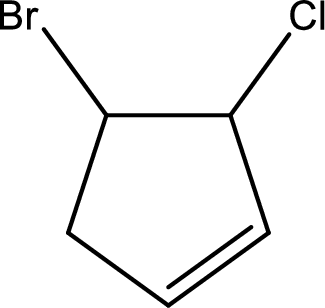
Given compound is found to contain five carbon atoms in a cyclic ring structure. Therefore, the parent cycloalkane is cyclopentane. As there is a double bond present in the ring, the parent compound name is cyclopentene.
Numbering has to be given in a way that the double bond gets the least numbering and also the substituents. In this case, double bond is present between carbon-1 and carbon-2.
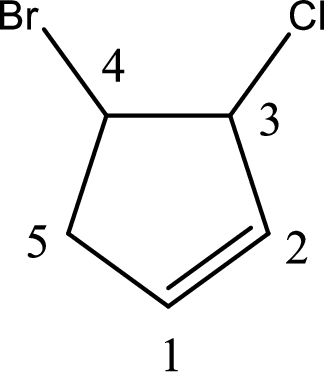
The substituents present in the given structure are a chlorine atom on carbon-3 and a bromine atom on carbon-4. Substituents have to be arranged in alphabetical order. Number has to be added before the substituent names which indicate the carbon atom in which it is present. This gives the name of compound as 4-bromo-3-chlorocyclopentene.
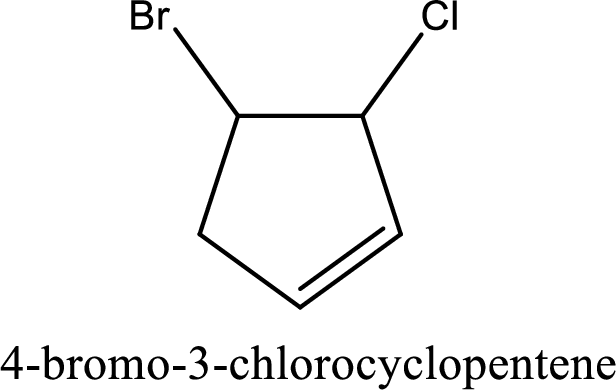
Parent chain is found to be cyclopentene. Position of double bond is carbon-1. Substituent present in the chain is 4-bromo-3-chloro. IUPAC name of the compound given is 4-bromo-3-chlorocyclopentene.
Want to see more full solutions like this?
Chapter 11 Solutions
GENERAL,ORGANIC+BIOCHEM (LOOSELEAF)
- Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure Yes. Is the proposed Lewis structure reasonable? Cl- : 2: :Z: :Z: N—N : 0: C C1: O CO No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0". ☑arrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions ΔΗ is (pick one) A This reaction is faster above 103. °C than below. AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous only above -9. °C. AS is (pick one) ΔΗ is (pick one) C The reverse of this reaction is always spontaneous. AS is (pick one) 18 Ararrow_forwardUse the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A The reverse of this reaction is always spontaneous but proceeds slower at temperatures below 41. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 94. °C. AS is (pick one) This reaction is always spontaneous, but ΔΗ is (pick one) C proceeds slower at temperatures below −14. °C. AS is (pick one) Х 00. 18 Ar 무ㅎ B 1 1arrow_forward
- Draw the product of the reaction shown below. Ignore inorganic byproducts. + H CH3CH2OH HCI Drawingarrow_forwardplease explain this in simple termsarrow_forwardK Most Reactive Na (3 pts) Can the metal activity series (shown on the right) or a standard reduction potential table explain why potassium metal can be prepared from the reaction of molten KCI and Na metal but sodium metal is not prepared from the reaction of molten NaCl and K metal? Show how (not). Ca Mg Al с Zn Fe Sn Pb H Cu Ag Au Least Reactivearrow_forward
- (2 pts) Why is O2 more stable as a diatomic molecule than S2?arrow_forwardDraw the Lewis structure for the polyatomic phosphite (PO¾³¯) a anion. Be sure to include all resonance structures that satisfy the octet rule. C I A [ ]¯arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. :0: Cl C C1: 0=0: : 0 : : 0 : H C N No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. ☐ No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forward
- Draw the Lewis structure for the polyatomic trisulfide anion. Be sure to include all resonance structures that satisfy the octet rule. с [ ] - Garrow_forward1. Calculate the accurate monoisotopic mass (using all 1H, 12C, 14N, 160 and 35CI) for your product using the table in your lab manual. Don't include the Cl, since you should only have [M+H]*. Compare this to the value you see on the LC-MS printout. How much different are they? 2. There are four isotopic peaks for the [M+H]* ion at m/z 240, 241, 242 and 243. For one point of extra credit, explain what each of these is and why they are present. 3. There is a fragment ion at m/z 184. For one point of extra credit, identify this fragment and confirm by calculating the accurate monoisotopic mass. 4. The UV spectrum is also at the bottom of your printout. For one point of extra credit, look up the UV spectrum of bupropion on Google Images and compare to your spectrum. Do they match? Cite your source. 5. For most of you, there will be a second chromatographic peak whose m/z is 74 (to a round number). For one point of extra credit, see if you can identify this molecule as well and confirm by…arrow_forwardPlease draw, not just describe!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





