
Concept explainers
The following names are incorrect, according to IUPAC rules. Draw the structural formulas and tell why each name is incorrect. Write the correct name for each compound.
a.
b.
c.
d.
(a)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
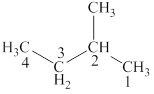
The longest chain in the given compound has four carbon atoms and one methyl group is attached to the second carbon. The correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
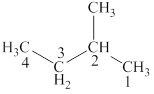
Figure 1
The structural formulas for given compound is shown in Figure 1. The longest chain in the given compound has four carbon atoms and one methyl group is attached to the second carbon. The correct IUPAC name of the given compound is
(b)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
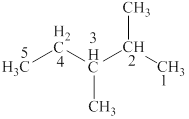
The lowest possible number is given to the carbon at which a group is attached. Thus, the correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
The lowest possible number is given to the carbon from which a group is attached. Thus, the correct IUPAC name of the given compound is

Figure 2
The structural formulas for given compound is shown in Figure 2. The lowest possible number is given to the carbon from which a group is attached. Thus, the correct IUPAC name of the given compound is
(c)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
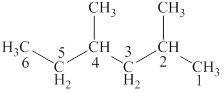
The parent chain is hexane and one methyl group each is attached to second and fourth carbon atom. Thus, the correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
The longest chain in the given compound has six carbon atoms. Thus, the parent chain is hexane. The lowest possible number is given to the carbon from which a group is attached. Thus, methyl group are attached to second and fourth carbon. Thus, the correct IUPAC name of the given compound is

Figure 3
The structural formulas for given compound is shown in Figure 3. The parent chain is hexane and one methyl group each is attached to second and fourth carbon atoms. Thus, the correct IUPAC name of the given compound is
(d)
Interpretation:
The structural formulas for given compound is to be drawn. The reason as to why the given compound name is incorrect is to be stated. The correct name for the given compound is to be stated.
Concept introduction:
The condensed structural formula of the compound represents the arrangement of atoms by showing specific covalent bonds. The molecular formula represents the atoms and their subscript represents the number of atoms without showing any covalent bonds. The structural formula represents the arrangement of atoms in space.
Answer to Problem 11.42E
The structural formulas for given compound is,
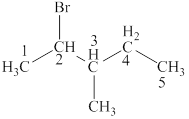
The parent chain is pentane and bromine and methyl group are attached to second and third carbon respectively. Thus, the correct IUPAC name of the given compound is
Explanation of Solution
The given compound is
The longest chain in the given compound has five carbon atoms. Thus, the parent chain is pentane. The lowest possible number is given to the carbon from which a group is attached. Thus, bromine and methyl group are attached to second and third carbon respectively. Thus, the correct IUPAC name of the given compound is

Figure 4
The structural formulas for given compound is shown in Figure 4. The parent chain ispentane and bromine and methyl group are attached to second and third carbon respectively. Thus, the correct IUPAC name of the given compound is
Want to see more full solutions like this?
Chapter 11 Solutions
Bundle: Chemistry For Today: General, Organic, And Biochemistry, 9th + Owlv2 With Mindtap Reader, 1 Term (6 Months) Printed Access Card
- Safari File Edit View History Bookmarks Window Help く < mylabmastering.pearson.com Wed Feb 12 8:44 PM ✩ + Apple Q Bing Google SignOutOptions M Question 36 - Lab HW BI... P Pearson MyLab and Mast... P Course Home Error | bartleby b Answered: If the biosynth... Draw a free-radical mechanism for the following reaction, forming the major monobromination product: ScreenPal - 2022 CHEM2... Access Pearson 2 CH3 Br-Br CH H3 Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron- flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. Include all free radicals by right-clicking on an atom on the canvas and then using the Atom properties to select the monovalent radical. ▸ View Available Hint(s) 0 2 DE [1] H EXP. CONT. H. Br-Br H FEB 12arrow_forwardPlease correct answer and don't use hand ratingarrow_forwardNonearrow_forwardQ1: For each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label each compound as chiral or achiral. + CI Br : Н OH H wo་ཡིག་ཐrow HO 3 D ။။ဂ CI Br H, CI Br Br H₂N OMe R IN I I N S H Br ជ័យ CI CI D OHarrow_forwardPlease correct answer and don't use hand ratingarrow_forwardNonearrow_forward%Reflectance 95 90- 85 22 00 89 60 55 50 70 65 75 80 50- 45 40 WA 35 30- 25 20- 4000 3500 Date: Thu Feb 06 17:21:21 2025 (GMT-05:0(UnknownD Scans: 8 Resolution: 2.000 3000 2500 Wavenumbers (cm-1) 100- 2981.77 1734.25 2000 1500 1000 1372.09 1108.01 2359.09 1469.82 1181.94 1145.20 1017.01 958.45 886.97 820.49 668.25 630.05 611.37arrow_forwardNonearrow_forwardCH3 CH H3C CH3 H OH H3C- -OCH2CH3 H3C H -OCH3 For each of the above compounds, do the following: 1. List the wave numbers of all the IR bands in the 1350-4000 cm-1 region. For each one, state what bond or group it represents. 2. Label equivalent sets of protons with lower-case letters. Then, for each 1H NMR signal, give the 8 value, the type of splitting (singlet, doublet etc.), and the number protons it represents. of letter δ value splitting # of protons 3. Redraw the compound and label equivalent sets of carbons with lower-case letters. Then for each set of carbons give the 5 value and # of carbons it represents. letter δ value # of carbonsarrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





