
Concept explainers
(a)
Interpretation:
The detailed mechanism for the production of given compounds from the respective alkyne is to be drawn.
Concept introduction:
The
Answer to Problem 11.39P
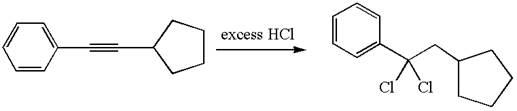
The detailed mechanism for the given reaction with a major product is:
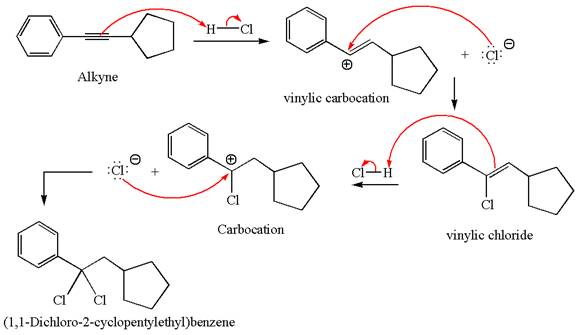
Explanation of Solution
The structure for the given compound
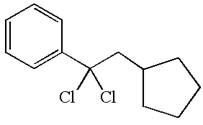
The given compound is germinal dichloride, and thus can be produced by electrophilic addition of an excess of
In the given reaction the alkyne is the electron rich site and the hydrogen from the
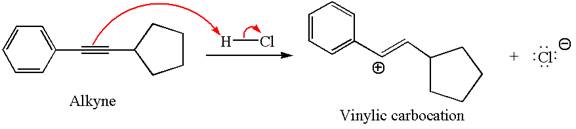
In the second step, the chloride ion acts as a nucleophile and attacks at vinylic carbocation forming vinylic chloride product.
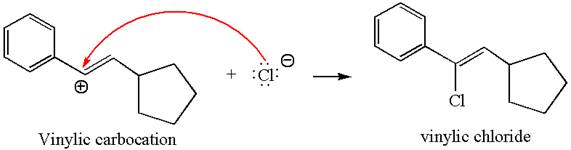
The vinylic chloride again undergoes the addition of
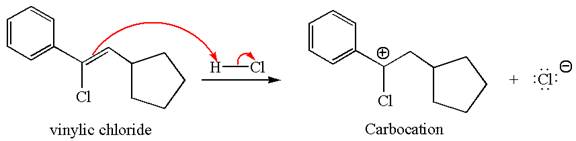
This carbocation further reacts with nucleophilic chloride ion to form a germinal dichloride product.
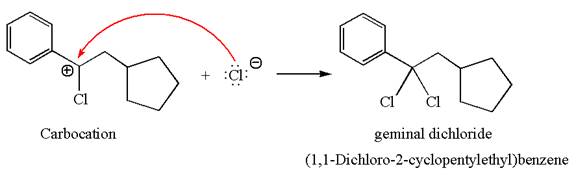
The detailed mechanism is drawn for the given reaction with showing the formation of stable carbocations and major product.
(b)
Interpretation:
The detailed mechanism for the production of given compounds from respective alkyne is to be drawn.
Concept introduction:
The alkynes are electron rich system like alkenes and can undergo an electrophilic addition reaction with strong Bronsted acids just like the alkenes do. The reaction proceeds with proton transfer reaction to form a stable carbocation followed by the action of water as a nucleophile. In excess of reagent, the reaction occurs twice forming a geminal dihalide compound as a major product.
Answer to Problem 11.39P
The detailed mechanism for the given reaction with the major product is:
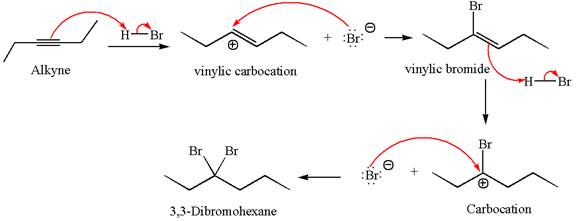
Explanation of Solution
The structure for the given compound
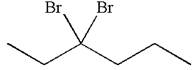
The given compound is germinal dibromide, thus can be produced by electrophilic addition of an excess of
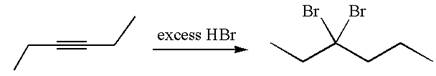
In the given reaction the alkyne is the electron rich site and the hydrogen from the
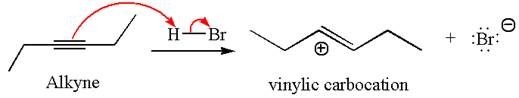
In the second step, the bromide ion acts as a nucleophile and attacks at vinylic carbocation forming vinylic bromide product.
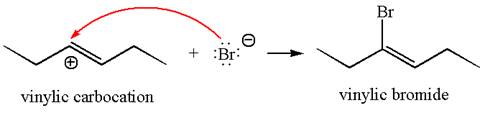
The vinylic bromide again undergoes the addition of
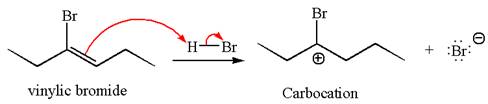
This carbocation further reacts with nucleophilic bromide ion to form germinal dibromide product.
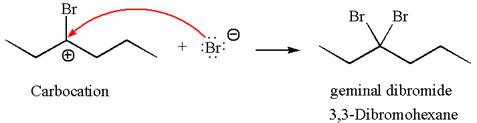
The detailed mechanism is drawn for the given reaction with showing the formation of stable carbocations and major product.
(c)
Interpretation:
The detailed mechanism for the production of given compounds from respective alkyne is to be drawn.
Concept introduction:
The alkynes are electron rich system like alkenes and can undergo an electrophilic addition reaction with strong Bronsted acids just like the alkenes do. The reaction proceeds with proton transfer reaction to form a stable carbocation followed by the action of water as a nucleophile. In a single addition reaction, the reaction occurs only once forming a vibylic halide compound as a major product. The deuterium is an isotope of a hydrogen atom and reacts the same as hydrogen.
Answer to Problem 11.39P
The detailed mechanism for the given reaction with a major product is:
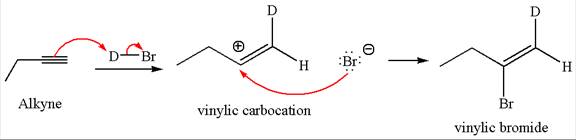
Explanation of Solution
The structure for the given compound is:
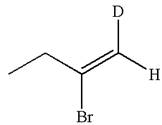
The given compound is vinylic bromide having deuterium at adjacent carbon, thus can be produced by single electrophilic addition of
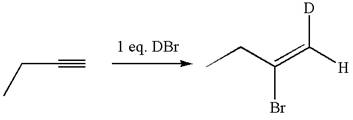
In the given reaction the alkyne is the electron rich site and the hydrogen from the
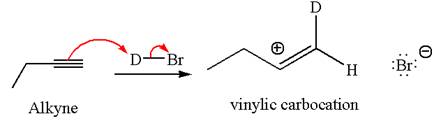
In the second step the bromide ion acts as a nucleophile and attacks at vinylic carbocation forming vinylic bromide product.
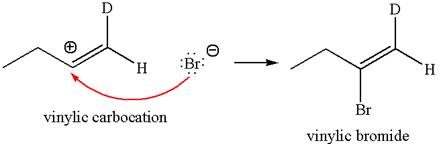
The detailed mechanism is drawn for the given reaction with showing the formation of stable carbocations and major product.
(d)
Interpretation:
The detailed mechanism for the production of given compounds from respective alkyne is to be drawn.
Concept introduction:
The alkynes are electron rich system like alkenes and can undergo an electrophilic addition reaction with strong Bronsted acids just like the alkenes do. The reaction proceeds with proton transfer reaction to form a stable carbocation followed by the action of water as a nucleophile. In excess of reagent, the reaction occurs twice forming a geminal dihalide compound as a major product.
Answer to Problem 11.39P
The detailed mechanism for the given reaction with the major product is:
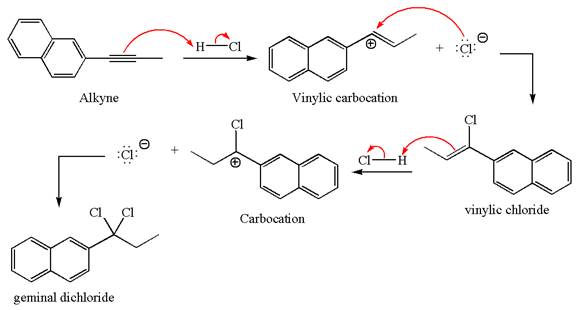
Explanation of Solution
The structure for the given compound is:
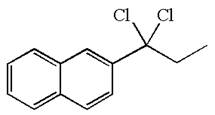
The given compound is germinal dichloride, thus can be produced by electrophilic addition of an excess of
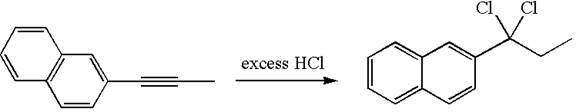
In the given reaction the alkyne is the electron rich site and the hydrogen from the
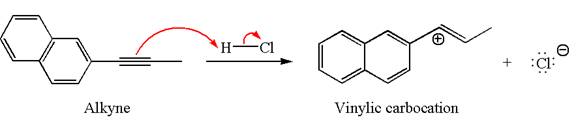
In the second step, the chloride ion acts as a nucleophile and attacks at vinylic carbocation forming vinylic chloride products.
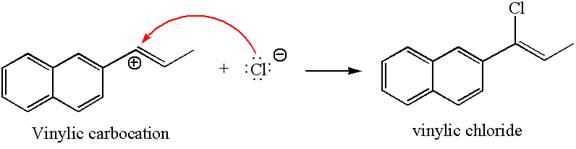
The vinylic chloride again undergoes the addition of
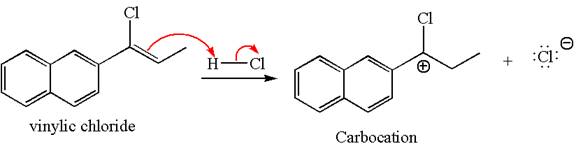
This carbocation further reacts with nucleophilic chloride ion to form a germinal dichloride product.
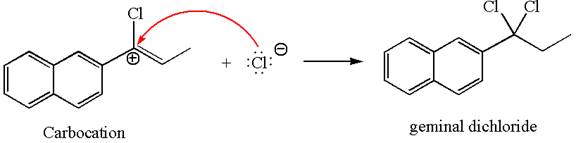
The detailed mechanism is drawn for the given reaction with showing the formation of stable carbocations and major product.
Want to see more full solutions like this?
Chapter 11 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- How many chiral centers are there in the following molecule? HO 0 1 ○ 2 ♡ 4 'N'arrow_forwardThe following chemical structure represents a molecule of what molecular formula?arrow_forwardWhich region(s) of the following phospholipid is/are hydrophobic? RO I hydro-water phobic-dislikes = Hydrophobic dislikes water ○ I only Il only I and III only II and IV only O II, III, and IV only III || IVarrow_forward
- Given the following data, determine the order of the reaction with respect to H2. H2(g) + 21Cl(g) → I2(g) + 2HCl(g) Experiment [H2] (torr) [ICI] (torr) Rate (M/s) 1 250 325 0.266 2 250 81 0.0665 3 50 325 0.266arrow_forwardWhich one of the following molecules is chiral? H- NH₂ H3C དང་།་ OH H HO H₂N HO- -H CHO -OH H HO- OH H- -H CH₂OH OHarrow_forwardThe structure of an unsaturated phospholipid is shown below. Which region of the molecule is most hydrophilic ? H₂N-CH₂ H₂C IV CH3 CH3 hydro-water philic-likes = Hydrophilic likes water ○ IV All regions are equally hydrophilic. IIIarrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


