
(a)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(a)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
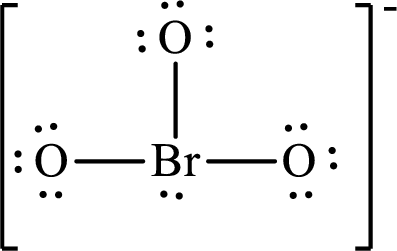
Boron forms three single bonds with three oxygen atoms and one lone pair is present on it so four hybrid orbitals are required and therefore the hybridization of boron in
The presence of lone pair on the central atom results in the deviation of bond angles from that of the ideal ones.
(b)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(b)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
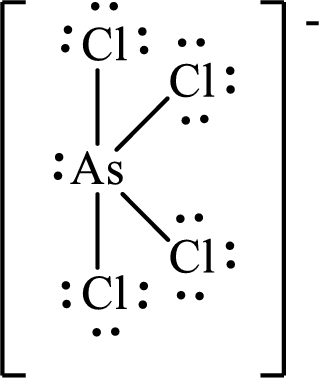
Arsenic forms four single bonds with four chlorine atoms and one lone pair is present on it so five hybrid orbitals are required and therefore the hybridization of arsenic in
The presence of lone pair on the central atom results in the deviation of bond angles from that of the ideal ones.
(c)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(c)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
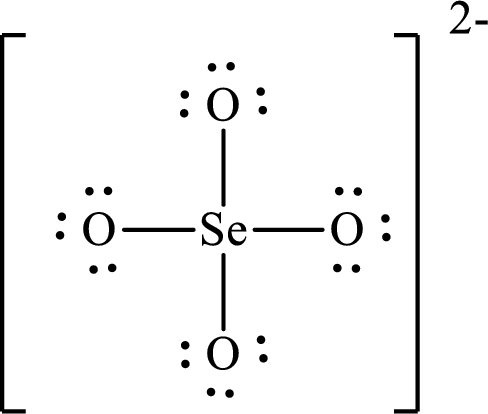
Selenium forms four single bonds with four oxygen atoms so four hybrid orbitals are required and therefore the hybridization of selenium in
The shape of the molecule is determined by the hybridization only if no lone pair is present on the central atom.
(d)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(d)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
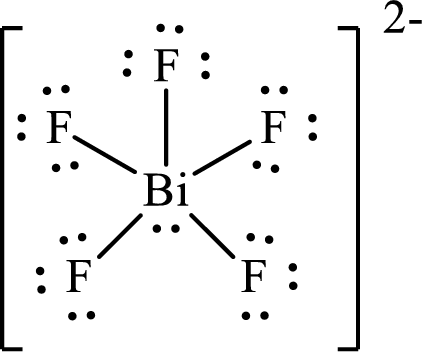
Bismuth forms five single bonds with five fluorine atoms and one lone pair is present on it so six hybrid orbitals are required and therefore the hybridization of bismuth in
The presence of lone pair on the central atom results in the deviation of bond angles from that of the ideal ones.
(e)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(e)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
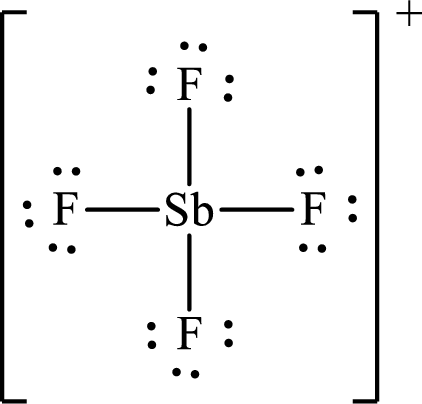
Antimony forms four single bonds with four fluorine atoms so four hybrid orbitals are required and therefore the hybridization of antimony in
The shape of the molecule is determined by the hybridization only if no lone pair is present on the central atom.
(f)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(f)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
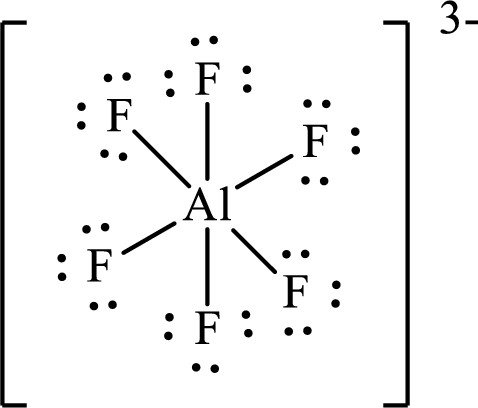
Aluminium forms six single bonds with six fluorine atoms so six hybrid orbitals are required and therefore the hybridization of aluminium in
The shape of the molecule is determined by the hybridization only if no lone pair is present on the central atom.
(g)
Interpretation:
The shape, hybridization of the central atom, ideal and deviated bond angle in
Concept introduction:
Hybridization is the process of intermixing of atomic orbital of slightly different energies to form hybrid orbitals that have similar energy. These orbital have lower energy and more stability than the atomic orbital. Hybridization of the central atom can be determined from the number of electron groups around the central atom in the Lewis structure of the molecule. Single bond, double bond, triple bond and lone pair all are considered as single electron group.
The shape of the molecule is determined by the electron bond pairs and lone pairs that are present around the central atom. The angle between the two bonds is called the bond angle. It is determined by the hybridization of the central atom and the presence of lone pairs around it.
(g)
Answer to Problem 11.38P
In
Explanation of Solution
The Lewis structure of
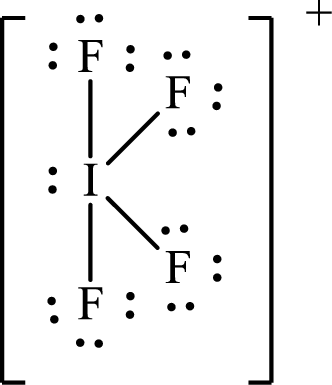
Iodine forms four single bonds with four fluorine atoms and one lone pair is present on it so five hybrid orbitals are required and therefore the hybridization of iodine in
The presence of lone pair on the central atom results in the deviation of bond angles from that of the ideal ones.
Want to see more full solutions like this?
Chapter 11 Solutions
CHEMISTRY >CUSTOM<
- 1. Identify the following alkenes as E or Z NH₂ Br 2. Draw the structures based on the IUPAC names (3R,4R)-3-bromo-4-fluoro- 1-hexene (Z)-4-bromo-2-iodo-3-ethyl- 3-heptene تر 3. For the following, predict all possible elimination product(s) and circle the major product. HO H₂SO4 Heat 80 F4 OH H2SO4 Heat 어요 F5 F6 1 A DII 4 F7 F8 F9 % & 5 6 7 * ∞ 8 BAB 3 E R T Y U 9 F D G H J K O A F11 F10arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. ○ O 1. H₂O, pyridine 2. neutralizing work-up a N W X 人 Parrow_forward✓ Check the box under each molecule that has a total of five ẞ hydrogens. If none of the molecules fit this description, check the box underneath the table. tab OH CI 0 Br xx Br None of these molecules have a total of five ẞ hydrogens. esc Explanation Check caps lock shift 1 fn control 02 F2 W Q A N #3 S 80 F3 E $ t 01 205 % 5 F5 & 7 © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility FT * 8 R T Y U כ F6 9 FIG F11 F D G H J K L C X V B < N M H option command P H + F12 commandarrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts and the carboxylic acid side product. O 1. CHзMgBr (excess) 2. H₂O ✓ W X 人arrow_forwardIf cyclopentyl acetaldehyde reacts with NaOH, state the product (formula).arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. N S S HgCl2, H2SO4 く 8 W X Parrow_forward
- tab esc く Drawing the After running various experiments, you determine that the mechanism for the following reaction occurs in a step-wise fashion. Br + OH + Using this information, draw the correct mechanism in the space below. 1 Explanation Check F2 F1 @2 Q W A os lock control option T S # 3 80 F3 Br $ 4 0105 % OH2 + Br Add/Remove step X C F5 F6 6 R E T Y 29 & 7 F D G H Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce A F7 DII F8 C Ո 8 * 9 4 F10 F C J K L C V Z X B N M H command P ge Coarrow_forwardIndicate compound A that must react with ethylbenzene to obtain 4-ethylbenzene-1-sulfonic acid. 3-bromo-4-ethylbenzene-1-sulfonic acid.arrow_forwardPart 1 of 2 Draw the structure of A, the minor E1 product of the reaction. esc I Skip Part Check H₂O, D 2 A + Click and drag to start drawing a structure. -0- F1 F2 1 2 # 3 Q A 80 F3 W E S D F4 $ 4 % 5 F5 ㅇ F6 R T Y F G X 5 & 7 + Save 2025 McGraw Hill LLC. All Rights Reserved. DII F7 F8 H * C 80 J Z X C V B N 4 F9 6arrow_forward
- File Preview The following is a total synthesis of the pheromone of the western pine beetle. Such syntheses are interesting both because of the organic chemistry, and because of the possibility of using species specific insecticides, rather than broad band insecticides. Provide the reagents for each step. There is some chemistry from our most recent chapter in this synthesis, but other steps are review from earlier chapters. (8 points) COOEt COOEt A C COOEt COOEt COOH B OH OTS CN D E See the last homework set F for assistance on this one. H+, H₂O G OH OH The last step is just nucleophilic addition reactions, taking the ketone to an acetal, intramolecularly. But it is hard to visualize the three dimensional shape as it occurs. Frontalin, pheromone of the western pine beetlearrow_forwardFor the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. C Major Product: Check + ◎ + X ง © Cl I F2 80 F3 I σ F4 I F5 NaOH Click and drawing F6 A 2025 McGraw Hill LLC. All Rights E F7 F8 $ # % & 2 3 4 5 6 7 8 Q W E R T Y U A S D F G H Jarrow_forwardCan I please get help with this graph. If you can show exactly where it needs to pass through.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





