
Concept explainers
Answer the following questions about erlotinib and phomallenic acid C. Erlotinib, sold under the trade name Tarceva, was introduced in 2004 for the treatment of lung cancer. Phomallenic acid C is an inhibitor of bacterial fatty acid synthesis.
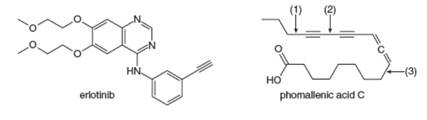
a. Which
b. What orbitals are used to form the shortest
c. Which H atom in phomallenic acid C is most acidic.
d. How many
e. Rank the labeled bonds in phomallenic acid C in order of increasing bond strength.
Want to see the full answer?
Check out a sample textbook solution
Chapter 11 Solutions
ORGANIC CHEMISTRY
Additional Science Textbook Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Fundamentals Of Thermodynamics
Human Biology: Concepts and Current Issues (8th Edition)
HUMAN ANATOMY
- Follow the curved arrows to draw a second resonance structure for each species. Explain and steps for individual understanding.arrow_forwardDraw all reasonable resonance structures for the following cation. Then draw the resonance hybrid. Provide steps and explanationarrow_forwardHow are the molecules or ions in each pair related? Classify them as resonance structures, isomers, or neither.arrow_forward
- How do I solve this Alkyne synthesis homework problem for my Organic Chemistry II class? I have to provide both the intermediate products and the reagents used.arrow_forwardSubstance X is known to exist at 1 atm in the solid, liquid, or vapor phase, depending on the temperature. Additionally, the values of these other properties of X have been determined: melting point enthalpy of fusion 90. °C 8.00 kJ/mol boiling point 130. °C enthalpy of vaporization 44.00 kJ/mol density 2.80 g/cm³ (solid) 36. J.K mol (solid) 2.50 g/mL (liquid) heat capacity 32. J.Kmol (liquid) 48. J.Kmol (vapor) You may also assume X behaves as an ideal gas in the vapor phase. Ex Suppose a small sample of X at 50 °C is put into an evacuated flask and heated at a constant rate until 15.0 kJ/mol of heat has been added to the sample. Graph the temperature of the sample that would be observed during this experiment. o0o 150- 140 130- 120- 110- 100- G Ar ?arrow_forwardMechanism. Provide the mechanism for the reaction below. You must include all arrows, intermediates, and formal charges. If drawing a Sigma complex, draw all major resonance forms. The ChemDraw template of this document is available on Carmen. Br FeBr3 Brarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning



