
Concept explainers
a)
Interpretation:
The IUPAC name of the
Concept introduction:
The longest continuous carbon chain in the molecule is chosen. The chain is numbered from the end which gives lowest number to the substituent either halo or alkyl group present. If different halogens are present they are numbered and listed in the alphabetical order while writing the name. If same two alternatives exist for different substituents, then the chain is numbered from the end that gives lowest number to the substituent that has alphabetical preference.
To give:
The IUPAC name of the alkyl halide shown.
Answer to Problem 14VC
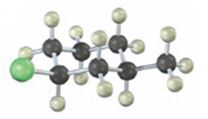
The alkyl halide given is
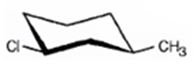
Its IUPAC name is cis- 1- chloro-3-methylcyclohexane.
Explanation of Solution
The compound has a cyclohexane ring with a Cl atom on C1 and a methyl on C3 both at equatorial positions. Hence its name is cis- 1- chloro-3-methylcyclohexane.
The IUPAC name of the alkyl halide shown is cis- 1- chloro-3-methylcyclohexane.
b)
Interpretation:
The IUPAC name of the alkyl halide is to be given.
Concept introduction:
The longest continuous carbon chain in the molecule is chosen. The chain is numbered from the end which gives lowest number to the substituent either halo or alkyl group present. If different halogens are present they are numbered and listed in the alphabetical order while writing the name. If same two alternatives exist for different substituents, then the chain is numbered from the end that gives lowest number to the substituent that has alphabetical preference.
To give:
The IUPAC name of the alkyl halide shown.
Answer to Problem 14VC
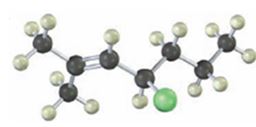
The alkyl halide given is
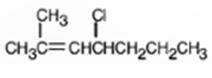
Its IUPAC name is 4-chloro-2-methyl-2-heptene.
Explanation of Solution
The compound has a seven carbon straight chain with a double bond between C2 & C3, a Cl atom on C4 and a methyl on C3. Hence its name is 4-chloro-2-methyl-2-heptene.
The IUPAC name of the alkyl halide shown is 4-chloro-2-methyl-2-heptene.
Want to see more full solutions like this?
Chapter 10 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
- Can you explain how I get these here and show the steps plz?arrow_forwardGive the IUPAC name for this compound Hydrocarbon Condensed Formulas Hint C2H5 CH2CH3 expand that in all the formula Part A: (CH3)2CHCH(C2H5)CH2CH2CH3 Give the IUPAC name for this compound. Part B: CH2=C(C2H5)CH2CH2CH3 Give the IUPAC name for this compound. Part C: (CH3)2C=CHC(C2H5)=CH2 Give the IUPAC name for this compound. Part D: CH3C=CCH(C2H5)2 Give the IUPAC name for this compound. Part E: (CH3)3CC=CCH2CH=C(CH3)2arrow_forwardSelect/ Match the correct letter from the image below for the IUPAC names given below: A B C D 3 E F G H K L Part 1. 4-methylheptane For example.mmmm Answer Letter H _for part 1 Part 2. 2,4-dimethylhexane Part 3. 2,3-dimethylpentane Part 4. 2,2-dimethylhexane Part 5. 2-ethyl-1,1,3,3-tetramethylcyclopentane Part 6. 3-ethyl-2-methylpentanearrow_forward
- Can u show the process as to how to get these?arrow_forwardSketch the expected 'H NMR spectra for the following compound. Label all of the H's in the structure and the corresponding signal for the spectra you sketch. Make sure you include the integration value and the splitting pattern for each signal Indicate how many signals you would expect in the 13C NMRarrow_forwardUse IUPAC naming rules to name the following hydrocarbon compounds: CH2-CH3 | a) CH-CH-CH2-CH-CH-CH3 b) | CH2 CH3 | CH3 CH3 \ / C=C H 1 H CH2-CH3 c) d) CH=C-CH3 e) CH3-CH2-CH2-CH=CH-CH3 f) CH2=CH-CH2-CH=CH-CH3 g) CH3-CH2-C = C-CH2-CH3 h)arrow_forward
- Q5 Name the following : a. b. C. d. e.arrow_forward25. Predict the major product of the following reaction. 1 equivalent of each of the starting materials was used. H₂C CH3 CH3 H3C H3C H3C. CH2 + H3C. heat CH3 CH H.C. CH3 H.C H.C CH3 CH CH3 CH3 A B C Earrow_forwardFind chemical structures based on the below information. a) Chemical formula C6H8O Compound is aromatic plus has two 1H NMR peaks that integrated for 3 each that are singlets (it could have more peaks in the 1H NMR b) Chemical Formula: C6H100 Compounds is conjugated 'H NMR has a signal that integrates for 6 and is a doublet IR spectra has a signal at 1730 cm-1arrow_forward
- Jaslev Propose a synthesis of the following starting from benzene and any other reagents and chemicals. No mechanisms are required. Indicate the condition for each step plus the major product for each step. More than two steps are required. Step 1 Step 2 مہد Brarrow_forwardPart C: The line formula for another branched alkane is shown below. i. In the IUPAC system what is the root or base name of this compound? ii. How many alkyl substituents are attached to the longest chain? iii. Give the IUPAC name for this compound.arrow_forwardPart D: Draw the Structural Formula for 4-ethyl-2-methylhexane Part E. Draw the Structural Formula for 1-chloro-3,3-diethylpentane (Chloro = Cl)arrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

