
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
9th Edition
ISBN: 9781305922198
Author: John E. McMurry
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.SE, Problem 44AP
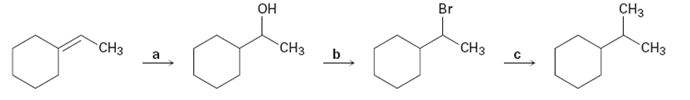
Identify the reagents a–c in the following scheme:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
C
app.aktiv.com
Draw the product of the following reaction sequence. Ignore
any inorganic byproducts formed.
H
O
1. (CH3CH2)2CuLi, THF
2. CH3Br
Drawing
Draw the product of the following reaction sequence. Ignore
any inorganic byproducts formed.
H
O
1. (CH3CH2)2CuLi, THF
2. CHзBr
Drawing
Seee the attached ima
Chapter 10 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
Ch. 10.1 - Prob. 1PCh. 10.1 - Draw structures corresponding to the following...Ch. 10.2 - Prob. 3PCh. 10.2 - Taking the relative reactivities of 1°, 2°, and...Ch. 10.4 - Prob. 5PCh. 10.4 - The major product of the reaction of...Ch. 10.4 - Prob. 7PCh. 10.5 - Prob. 8PCh. 10.6 - Prob. 9PCh. 10.6 - How might you replace a halogen substituent by a...
Ch. 10.7 - How would you carry out the following...Ch. 10.8 - Rank both sets of compounds in order of increasing...Ch. 10.8 - Tell whether each of the following reactions is an...Ch. 10.SE - Prob. 14VCCh. 10.SE - Prob. 15VCCh. 10.SE - Prob. 16VCCh. 10.SE - Draw the electron-pushing mechanism for each...Ch. 10.SE - Draw the electron-pushing mechanism for the...Ch. 10.SE - The formation of Br2 from NBS first involves the...Ch. 10.SE - In light of the fact that tertiary alkyl halides...Ch. 10.SE - Alkyl halides can be reduced to alkanes by a...Ch. 10.SE - Name the following alkyl halides:Ch. 10.SE - Prob. 23APCh. 10.SE - Draw and name all of the monochlorination products...Ch. 10.SE - How would you prepare the following compounds,...Ch. 10.SE - Prob. 26APCh. 10.SE - A chemist requires a large amount of...Ch. 10.SE - What product(s) would you expect from the reaction...Ch. 10.SE - What product(s) would you expect from the reaction...Ch. 10.SE - What product would you expect from the reaction of...Ch. 10.SE - Rank the compounds in each of the following series...Ch. 10.SE - Which of the following compounds have the same...Ch. 10.SE - Tell whether each of the following reactions is an...Ch. 10.SE - Prob. 34APCh. 10.SE - Alkylbenzenes such as toluene (methylbenzene)...Ch. 10.SE - Prob. 36APCh. 10.SE - Prob. 37APCh. 10.SE - Prob. 38APCh. 10.SE - Prob. 39APCh. 10.SE - Prob. 40APCh. 10.SE - The syntheses shown here are unlikely to occur as...Ch. 10.SE - Why do you suppose its not possible to prepare a...Ch. 10.SE - Prob. 43APCh. 10.SE - Identify the reagents a–c in the following...Ch. 10.SE - Prob. 45APCh. 10.SE - Prob. 46APCh. 10.SE - Prob. 47APCh. 10.SE - The relative rate of radical bromination is...Ch. 10.SE - Prob. 49APCh. 10.SE - Predict the product and provide the entire...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Look at the image attached pleaarrow_forwardComplete the mechanismarrow_forwardV Biological Macromolecules Drawing the Haworth projection of an aldose from its Fischer projection Draw a Haworth projection of a common cyclic form of this monosaccharide: H C=O HO H HO H H OH CH₂OH Explanation Check Click and drag to start drawing a structure. Xarrow_forward
- Complete the mechanismarrow_forwardComplete the mechanismarrow_forward8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License