
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
9th Edition
ISBN: 9781305922198
Author: John E. McMurry
Publisher: CENGAGE L
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.SE, Problem 42AP
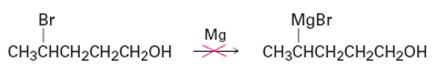
Why do you suppose it’s not possible to prepare a Grignard reagent from a bromo alcohol such as 4-bromo-1-pentanol? Give another example of a molecule that is unlikely to form a Grignard reagent.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Write the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophen
For the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. 
How to draw the reaction mechasnism below
Chapter 10 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
Ch. 10.1 - Prob. 1PCh. 10.1 - Draw structures corresponding to the following...Ch. 10.2 - Prob. 3PCh. 10.2 - Taking the relative reactivities of 1°, 2°, and...Ch. 10.4 - Prob. 5PCh. 10.4 - The major product of the reaction of...Ch. 10.4 - Prob. 7PCh. 10.5 - Prob. 8PCh. 10.6 - Prob. 9PCh. 10.6 - How might you replace a halogen substituent by a...
Ch. 10.7 - How would you carry out the following...Ch. 10.8 - Rank both sets of compounds in order of increasing...Ch. 10.8 - Tell whether each of the following reactions is an...Ch. 10.SE - Prob. 14VCCh. 10.SE - Prob. 15VCCh. 10.SE - Prob. 16VCCh. 10.SE - Draw the electron-pushing mechanism for each...Ch. 10.SE - Draw the electron-pushing mechanism for the...Ch. 10.SE - The formation of Br2 from NBS first involves the...Ch. 10.SE - In light of the fact that tertiary alkyl halides...Ch. 10.SE - Alkyl halides can be reduced to alkanes by a...Ch. 10.SE - Name the following alkyl halides:Ch. 10.SE - Prob. 23APCh. 10.SE - Draw and name all of the monochlorination products...Ch. 10.SE - How would you prepare the following compounds,...Ch. 10.SE - Prob. 26APCh. 10.SE - A chemist requires a large amount of...Ch. 10.SE - What product(s) would you expect from the reaction...Ch. 10.SE - What product(s) would you expect from the reaction...Ch. 10.SE - What product would you expect from the reaction of...Ch. 10.SE - Rank the compounds in each of the following series...Ch. 10.SE - Which of the following compounds have the same...Ch. 10.SE - Tell whether each of the following reactions is an...Ch. 10.SE - Prob. 34APCh. 10.SE - Alkylbenzenes such as toluene (methylbenzene)...Ch. 10.SE - Prob. 36APCh. 10.SE - Prob. 37APCh. 10.SE - Prob. 38APCh. 10.SE - Prob. 39APCh. 10.SE - Prob. 40APCh. 10.SE - The syntheses shown here are unlikely to occur as...Ch. 10.SE - Why do you suppose its not possible to prepare a...Ch. 10.SE - Prob. 43APCh. 10.SE - Identify the reagents a–c in the following...Ch. 10.SE - Prob. 45APCh. 10.SE - Prob. 46APCh. 10.SE - Prob. 47APCh. 10.SE - The relative rate of radical bromination is...Ch. 10.SE - Prob. 49APCh. 10.SE - Predict the product and provide the entire...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the following molecules with IUpacarrow_forwardWhat is the molecular orbital for cyclopropenyl anion and is it aromatic, antiaromatic or nonaromatic?arrow_forwardUsing the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forward
- How to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forwardWrite the Esterification reaction mechanism for acetic acid, and one propanol to make propanol ethanoate (molecule that gives peas its odor in flavor)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY