
The gas-turbine portion of a combined gas–steam power plant has a pressure ratio of 16. Air enters the compressor at 300 K at a rate of 14 kg/s and is heated to 1500 K in the combustion chamber. The combustion gases leaving the gas turbine are used to heat the steam to 400°C at 10 MPa in a heat exchanger. The combustion gases leave the heat exchanger at 420 K. The steam leaving the turbine is condensed at 15 kPa. Assuming all the compression and expansion processes to be isentropic, determine (a) the mass flow rate of the steam, (b) the net power output, and (c) the thermal efficiency of the combined cycle. For air, assume constant specific heats at room temperature.
(a)
The mass flow rate of the steam.
Answer to Problem 77P
The mass flow rate of the steam is
Explanation of Solution
Show the
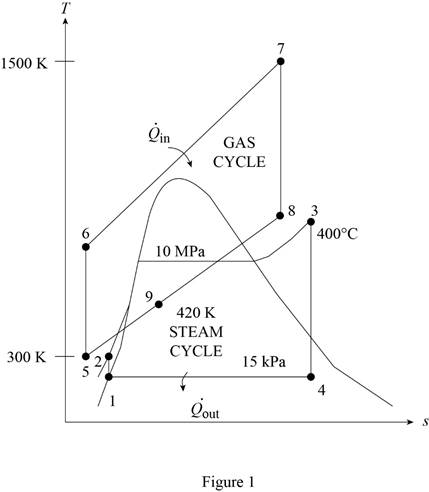
Determine the temperature of gas cycle at state 6.
Here, the temperature of gas cycle at state 5 is
Determine the rate of heat transfer into the gas turbine.
Here, the mass flow rate of air is
Determine the power rate for compressor of gas turbine.
Determine the temperature of gas cycle at state 8.
Here, the pressure of gas cycle at state 8 is
Determine the power rate for gas turbine of gas turbine.
Determine the net power output of the gas cycle.
Determine input work done per unit mass of the isentropic process for the steam cycle.
Here, the specific volume of the steam is
Determine the specific enthalpy at state 2 of the steam cycle.
Here, the specific enthalpy at the state 1 of the steam cycle is
Determine the quality at state 4 of the stream cycle.
Here, the specific entropy at state 4 is
Determine the specific enthalpy at state 4 of the steam cycle.
Here, the specific enthalpy of saturated liquid is
Write the expression for the steady-flow energy balance equation.
Here, the total energy rate of entering the system is
Substitute
Here, the temperature of gas cycle at state 8 is
Determine the power rate for gas turbine of steam cycle.
Here, the mass flow rate of the steam is
Determine the power rate of the isentropic process for the steam cycle.
Here, the mass flow rate of the steam is
Determine the net power output of the steam cycle.
Conclusion:
From the Table A-2, “Ideal-gas specific heats of various common gases”, obtain the value of specific heat of constant pressure and the ratio of specific heat at temperature of
Substitute 300 K for
Substitute
Substitute
Substitute 1500 K for
Substitute
Substitute 11547 kW for
From the Table A-4, “Saturated water-Pressure table”, obtain the value of the initial specific enthalpy at liquid state, specific volume at the liquid state, the specific entropy at liquid state, the specific enthalpy change upon vaporization at pressure, and the specific entropy change upon vaporization at pressure of 15 kPa as:
Substitute
Substitute
From the Table A-6, “Superheated water”, obtain the value of the specific enthalpy at state 3 and the specific entropy at state 3 at pressure of 10 MPa and temperature of
Substitute
Substitute 0.7528 for
Substitute
Thus, the mass flow rate of the steam is
Substitute
Substitute
Substitute 1384.013 kW for
(b)
The net work output of the combined cycle.
Answer to Problem 77P
The net work output of the combined cycle is
Explanation of Solution
Determine the net power output of combined cycle.
Conclusion:
Substitute 1371 kW for
Thus, the net work output of the combined cycle is
(c)
The thermal efficiency of the combined cycle.
Answer to Problem 77P
The thermal efficiency of the combined cycle is
Explanation of Solution
Determine the thermal efficiency of the combined cycle.
Conclusion:
Substitute 7819 kW for
Thus, the thermal efficiency of the combined cycle is
Want to see more full solutions like this?
Chapter 10 Solutions
THERMODYNAMICS(SI UNITS,INTL.ED)EBOOK>I
- The container truck engine operated on the diesel cycle with a compression ratio of 12. Assume the mass of air in the engine is conserved. At the start of the compression process, the air is at 1 atm and 30 oC. 400 kJ/kg of heat is removed from the air during the constant-volume heat rejection process. The ratio of . Through the diesel cycle, 20% of the work done by the air is used to operate the vehicle's refrigeration and heat pump system. One diesel cycle took 0.7s to complete. The heat rejection from the refrigeration and heat pump system is 800 kJ. The refrigeration system uses R-134a as the working fluid and operates between 100 kPa and 1200 kPa pressure limits. cp = 1.005 kJ/kg/K, cv = 0.7177 kJ/kg/K, R = 8.314J/mol/K, Molecular mass of air= 29g/mol. 1) Calculate the temperature at the start of the heat rejection process. 2) Calculate the temperature at the end of the heat addition process. 3) Calculate the temperature at the start of the heat addition process. 4) Calculate…arrow_forwardThe container truck engine operated on the diesel cycle with a compression ratio of 12. Assume the mass of air in the engine is conserved. At the start of the compression process, the air is at 1 atm and 30 oC. 400 kJ/kg of heat is removed from the air during the constant-volume heat rejection process. The ratio of V3/V4 is 0.2 Through the diesel cycle, 20% of the work done by the air is used to operate the vehicle's refrigeration and heat pump system. One diesel cycle took 0.7s to complete. The heat rejection from the refrigeration and heat pump system is 800 kJ. The refrigeration system uses R-134a as the working fluid and operates between 100 kPa and 1200 kPa pressure limits. cp = 1.005 kJ/kg/K, cv = 0.7177 kJ/kg/K, R = 8.314J/mol/K, Molecular mass of air= 29g/mol. Calculate the temperature at the start of the heat rejection process.arrow_forwardA gas turbine uses two compression and two expansion stages, each stage having a pressure ratio of 4. The working fluid is intercooled between the two compression stages and reheated between the two expansion stages. Air enters the gas turbine at 100kPa and 17°C. The combustion chamber and reheat stage each contribute 300kJ/kg of heat. A regenerator uses exhausted gases to increase the working fluid temperature prior to the combustion chamber by 20°C. Assume constant thermal properties of air evaluated at 300K during your solution. Assume all turbine and compressor stages are isentropic. Draw the T-s diagram based on the numbering convention in the schematic below. Determine the system's thermal efficiency. Determine the required air mass flow rate to obtain an output of 10MW. 26 REMEWS REHEAT JNTER. Ti C2 Come REGEN. Scannad wim Camirwnerarrow_forward
- The container truck engine operated on the diesel cycle with a compression ratio of 12. Assume the mass of air in the engine is conserved. At the start of the compression process, the air is at 1 atm and 30 oC. 400 kJ/kg of heat is removed from the air during the constant-volume heat rejection process. The ratio of v3/v4 is 0.2. Through the diesel cycle, 20% of the work done by the air is used to operate the vehicle's refrigeration and heat pump system. One diesel cycle took 0.7s to complete. The heat rejection from the refrigeration and heat pump system is 800 kJ. The refrigeration system uses R-134a as the working fluid and operates between 100 kPa and 1200 kPa pressure limits. cp = 1.005 kJ/kg/K, cv = 0.7177 kJ/kg/K, R = 8.314J/mol/K, Molecular mass of air= 29g/mol. a) Calculate the temperature at the start of the heat rejection process. b) Calculate the temperature at the end of the heat addition process. c) Calculate the temperature at the start of the heat addition process. d)…arrow_forwardSee the following pic:arrow_forwardQ11/ The gas-turbine portion of a combined gas-steam power plant has a pressure ratio of 16. Air enters the compressor at 300 K at a rate of 14 kg/s and is heated to 1500 K in the combustion chamber. The combustion gases leaving the gas turbine are used to heat the steam to 400°C at 10 MPa in a heat exchanger. The combustion gases leave the heat exchanger at 420 K. The steam leaving the turbine is condensed at 15 kPa. Assuming all the compression and expansion processes to be isentropic, determine (a) the mass flow rate of the steam, (b) the net power output, and (c) the thermal efficiency of the combined cycle. For air, assume constant specific heats at room temperature. Answers:(a) 1.275 kg/s, (b) 7819 kW, (c) 66.4 percent.arrow_forward
- Steam leaves a turbine at 250 kPa and 0.85 dryness fraction after isentropic expansion. The turbine inlet pressure is 3 MPa. A boiler feed-water pump extracts the moisture in the 250 kPa wet exhaust and returns it to the boiler, leaving only dry steam to enter a lower-pressure turbine. It expands isentropically in this turbine to 4 kPa, and a second feed-water pump returns the condensate to the boiler. Ignoring the feed-water pump terms, calculate the ideal-cycle efficiency.arrow_forwardSuperheated water vapor enters the turbine at 5Mpa and 4000C. The water leaves the condenser as saturated liquid at a pressure of 30kPa, and the turbine efficiency is 91%. The net power output of the cycle is 100MW. Determine the thermal efficiency.arrow_forwardSteam enters the turbine of a cogeneration plant at 6 MPa and 550°C. One-third of the steam is extractedfrom the turbine at 1400 kPa pressure for process heating. The remaining steam continues to expand to20 kPa. The extracted steam is then condensed and mixed with feedwater at constant pressure and themixture is pumped to the boiler pressure of 6 MPa. The mass flow rate of steam through the boiler is 30kg/s. Disregarding any pressure drops and heat losses in the piping, and assuming the turbine and thepump to be isentropic, determine (a) the net power produced, (b) the utilization factor of the plant, (c)the exergy destruction associated with the process heating, and (d) the entropy generation associatedwith the process in the boiler.Assuming a source temperature of 1000 K and a sink temperature of 298 K.arrow_forward
- Steam at 2000 psia and 1000°F enters the high-pressure turbine of a reheat cycle and is expanded adiabatically and reversibly to 200 psia. The steam is then passed through the superheater, from where it exits at the 950°F temperature. The low-pressure turbine expands the steam reversibly and adiabatically to 1 psia. If 7 lbm/s of steam is used and the circulating pump is assumed to operate ideally, the power produced, the added heat rate, and the cycle efficiency are: a) 7049,76 hp ; 12124,5 Btu/s ; 42,2% b) 7400,77 hp ; 11553,7 Btu/s ; 45,1% c) 8350,08 hp ; 13089,6 Btu/s ; 49,8% d) 7944,76 hp ; 11834,2 Btu/s ; 47,6%arrow_forwardA hot process stream is cooled by indirect heat exchange with feedwater to a boiler, thereby producing saturated steam. Assume that the heat exchanger is isolated. The liquid feed water to the boiler enters at 50 °C, and leaves as saturated steam at 10000 kPa. The flow of the process stream is 1000 kmol/h, the molar enthalpy at the input is 2000 kJ/kmol, and the output enthalpy is 800 kJ/kmol. Calculate a) the flow rate (kg/h) of steam produced. b) If we had to produce water vapor by burning fuel, find its savings (gallons/h), if the heating power of the fuel is 144,000 Btu/gal. Note: make a table of degrees of freedom. Neglect changes in kinetic and potential energy.Data: Boiler feed water enthalpy Ĥ (kJ/kg) ≈ 4.19 T(°C):arrow_forwardTNB steam power plant in Genting Highlandsoperates on reheat Rankine cycleand has power net output of 50MW. Steam enters the turbine at 12MPa and 500 °C and it is cooled in the condenser at a pressure of 12kPa by running cooling water from the Bertam River through the tubesof condenser. Steam enters both stages of the turbine at b°C. If the moisture content of the steam at the exit of the low-pressure turbine is not to exceed 10 percent, show the cycle on a T-s diagram and determine;i.The pressure at which reheating takes place, ii.The thermal efficiency of the cycleandiii.Please justify how does the thermal efficiency of the cycle if the temperature to low turbine is increase to 600°C?arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





