
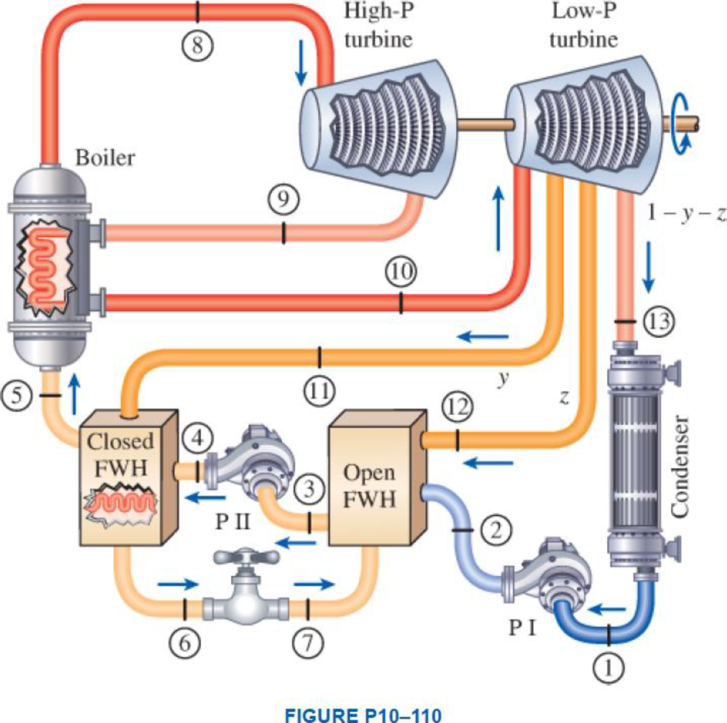
A steam power plant operates on an ideal reheat–regenerative Rankine cycle with one reheater and two feedwater heaters, one open and one closed. Steam enters the high-pressure turbine at 15 MPa and 600°C and the low-pressure turbine at 1 MPa and 500°C. The condenser pressure is 5 kPa. Steam is extracted from the turbine at 0.6 MPa for the closed feedwater heater and at 0.2 MPa for the open feedwater heater. In the closed feedwater heater, the feedwater is heated to the condensation temperature of the extracted steam. The extracted steam leaves the closed feedwater heater as a saturated liquid, which is subsequently throttled to the open feedwater heater. Show the cycle on a T-s diagram with respect to saturation lines. Determine (a) the fraction of steam extracted from the turbine for the open feedwater heater, (b) the thermal efficiency of the cycle, and (c) the net power output for a mass flow rate of 42 kg/s through the boiler.
(a)
The fraction of steam extracted from the turbine for the open feed water heater.
Answer to Problem 107RP
The fraction of steam extracted from the turbine for the open feed water heater is
Explanation of Solution
Draw the schematic layout of the given power plant that operates on an ideal reheat-regenerative Rankine cycle as shown in Figure 1.
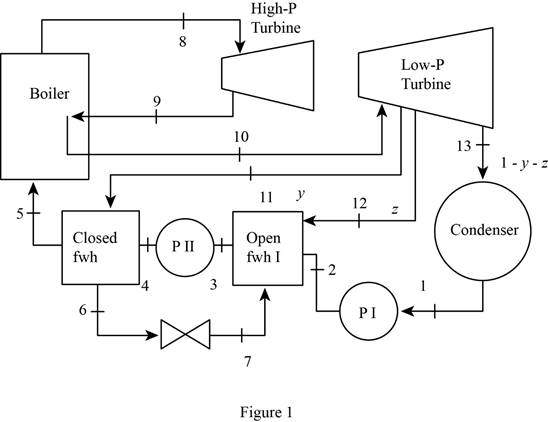
Draw the
Figure 2.
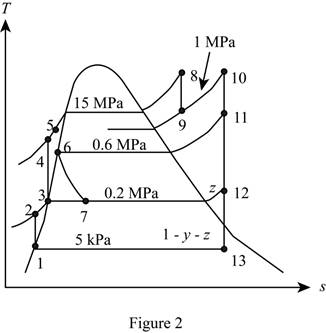
Here, water (steam) is the working fluid of the ideal regenerative Rankine cycle. The cycle involves two pumps.
Write the formula for work done by the pump during process 1-2.
Here, the specific volume is
Write the formula for enthalpy
Write the formula for work done by the pump during process 3-4.
Here, the specific volume is
Write the formula for enthalpy
Write the formula for enthalpy
The quality of water at state 13 is expressed as follows.
The enthalpy at state 13 is expressed as follows.
Here, the enthalpy is
Write the general equation of energy balance equation.
Here, the rate of net energy inlet is
At steady state the rate of change of net energy of the system
Refer Equation (VIII).
Write the energy balance equation for open feed water heater.
Here, the mass fraction steam extracted from the turbine to the inlet mass of the boiler
Rewrite the Equation (IX) in terms of mass fraction
For the open FWH,
Here, the mass fraction steam extracted from the turbine to the inlet mass of the boiler
Solving Equation (XI).
Conclusion:
At state 1:
The water exits the condenser as a saturated liquid at the pressure of
Refer Table A-5, “Saturated water-Pressure table”.
The enthalpy
Substitute
Substitute
From the Table A-5, “Saturated water-temperature Table” obtains the value of the enthalpy
Substitute
Substitute
From the Table A-5, “Saturated water-temperature Table” obtains the value of the enthalpy
Here, the temperature at the state 6
Substitute
From the Table A-6, “Superheated water” obtains the value of the enthalpy
From the Table A-6, “Superheated water” obtains the value of the enthalpy
Here, the entropy at the state 9
From the Table A-6, “Superheated water” obtains the value of the enthalpy
Refer Table A-6, “superheated water”, and write the enthalpy at state 11 at pressure of
Here, enthalpy of saturation liquid at pressure of
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is specific entropy and specific enthalpy at state 11 respectively.
Show the specific enthalpy at state 11 corresponding to temperature as in Table (1).
|
Specific entropy at state 11 |
Specific enthalpy at state 11 |
| 7.7097 | 3270.8 |
| 7.7642 | |
| 8.0041 | 3483.4 |
Substitute
Substitute
Similarly repeat the Equation (XIV) for specific enthalpy at state 11 corresponding to the pressure of
From the Table A-5, “Saturated water” obtains the value of the specific entropy of saturated liquid
Substitute
Substitute
Substitute
Substitute
Thus, the fraction of steam extracted from the turbine for the open feed water heater is
(b)
The thermal efficiency of the cycle.
Answer to Problem 107RP
The thermal efficiency of the cycle is
Explanation of Solution
Write the formula for heat in
Write the formula for net power output of the cycle per unit mass.
Write the formula for thermal efficiency of the cycle
Conclusion:
Substitute
Substitute 0.06215 for
Substitute
Substitute
Thus, the thermal efficiency of the cycle is
(c)
The net power output for mass flow rate of
Answer to Problem 107RP
The net power output for mass flow rate of
Explanation of Solution
Write the expression for net power output for mass flow rate of
Here, the mass flow rate through the boiler is
Conclusion:
Substitute
Thus, the net power output for mass flow rate of
Want to see more full solutions like this?
Chapter 10 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
Additional Engineering Textbook Solutions
Modern Database Management
Starting Out with Programming Logic and Design (5th Edition) (What's New in Computer Science)
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Mechanics of Materials (10th Edition)
Java: An Introduction to Problem Solving and Programming (8th Edition)
Fluid Mechanics: Fundamentals and Applications
- Generate the kinematic diagram of the following mechanisms using the given symbols. Then, draw their graphs and calculate their degrees of freedom (DoF) using Gruebler's formula. PUNTO 2. PUNTO 3. !!!arrow_forwardCreate a schematic representation of the following mechanisms using the given symbols and draw their graphs. Then, calculate their degrees of freedom (DoF) using Gruebler's formula. PUNTO 6. PUNTO 7.arrow_forwardhow the kinematic diagram of the following mechanisms would be represented using the given symbols? PUNTO 0. PUNTO 1. °arrow_forward
- Create a schematic representation of the following mechanisms using the given symbols and draw their graphs. Then, calculate their degrees of freedom (DOF) using Gruebler's formula. PUNTO 4. PUNTO 5. (0) Groundarrow_forwardDraw the graph of ALL the mechanisms and calculate their DoF using Gruebler's formula. PUNTO 0. PUNTO 1.arrow_forwardAn adjustable support. Construction designed to carry vertical load and is adjusted by moving the blue attachment vertically. The link is articulated at both ends (free to rotate) and can therefore only transmit power axially. Analytically calculate the force to which the link is subjected? Calculate analytically rated voltage in the middle of the link.? F=20kN Alpha 30 deg Rel 225 Mpans:5arrow_forward
- A swivel crane where the load is moved axially along the beam through the wagon to which the hook is attached. Round bar with a diameter of ∅30 mm. The support beam is articulated at both ends (free to rotate) and can therefore only transmit force axially. Calculate reaction force in the x-direction at point A? Calculate analytical reaction force in the y-direction of point A? Calculate nominal stress in the middle of the support beam?Lengt 5 mAlfa 25 degX=1.5mIPE300-steelmass:1000 kgarrow_forwardgot wrong answers help pleasearrow_forwardA crate weighs 530 lb and is hung by three ropes attached to a steel ring at A such that the top surface is parallel to the xy plane. Point A is located at a height of h = 42 in above the top of the crate directly over the geometric center of the top surface. Use the dimensions given in the table below to determine the tension in each of the three ropes. 2013 Michael Swanbom cc00 BY NC SA ↑ Z C b B У a D Values for dimensions on the figure are given in the following table. Note the figure may not be to scale. Variable Value a 30 in b 43 in 4.5 in The tension in rope AB is 383 x lb The tension in rope AC is 156 x lb The tension in rope AD is 156 x lbarrow_forward
- A block of mass m hangs from the end of bar AB that is 7.2 meters long and connected to the wall in the xz plane. The bar is supported at A by a ball joint such that it carries only a compressive force along its axis. The bar is supported at end B by cables BD and BC that connect to the xz plane at points C and D respectively with coordinates given in the figure. Cable BD is elastic and can be modeled as a linear spring with a spring constant k = 400 N/m and unstretched length of 6.34 meters. Determine the mass m, the compressive force in beam AB and the tension force in cable BC. Z C D (c, 0, d) (a, 0, b) A B y f m cc 10 BY NC SA 2016 Eric Davishahl x Values for dimensions on the figure are given in the following table. Note the figure may not be to scale. Variable Value a 8.1 m b 3.3 m с 2.7 m d 3.9 m e 2 m f 5.4 m The mass of the block is 68.8 The compressive force in bar AB is 364 × kg. × N. The tension in cable BC is 393 × N.arrow_forwardThe airplane weighs 144100 lbs and flies at constant speed and trajectory given by 0 on the figure. The plane experiences a drag force of 73620 lbs. 0 a.) If 11.3°, determine the thrust and lift forces = required to maintain this speed and trajectory. b.) Next consider the case where is unknown, but it is known that the lift force is equal to 7.8 times the quantity (Fthrust Fdrag). Compute the resulting trajectory angle and the lift force in this case. Use the same values for the weight and drag forces as you used for part a. 20. YAAY' Farag Ө Fthrust CC + BY NC SA 2013 Michael Swanbom Flift Fweight The lift force acts in the y' direction. The weight acts in the negative y direction. The thrust and drag forces act in the positive and negative x' directions respectively. Part (a) The thrust force is equal to 101,855 ☑ lbs. The lift force is equal to 141,282 ☑ lbs. Part (b) The trajectory angle 0 is equal to 7.31 ✓ deg. The lift force is equal to 143,005 ☑ lbs.arrow_forwardsimply supported beam has a concentrated moment M, applied at the left support and a concentrated force F applied at the free end of the overhang on the right. Using superposition, determine the deflection equations in regions AB and BC.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





