
EBK PHYSICS FOR SCIENTISTS & ENGINEERS
5th Edition
ISBN: 9780134296074
Author: GIANCOLI
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.7, Problem 1CE
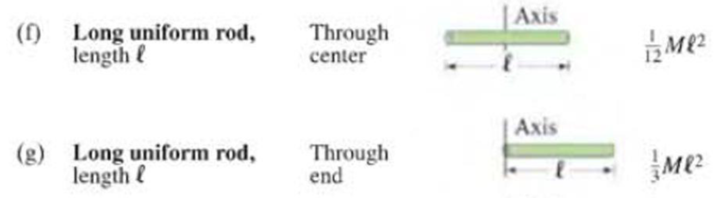
In Figs. 10–20f and g, the moments of inertia for a thin rod about two different axes are given. Are they related by the parallel-axis theorem? Please show how.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(a) Calculate the number of electrons in a small, electrically neutral silver pin that has a mass of 13.0 g. Silver has 47 electrons per atom, and its molar mass is 107.87 g/mol.
8
Two moving charged particles exert forces on each other because each creates a magnetic field that acts
on the other. These two "Lorentz" forces are proportional to vix (2 xr) and 2 x (vi x-r), where is the vector
between the particle positions. Show that these two forces are equal and opposite in accordance with Newton's third
law if and only if rx (vi × 2) = 0.
6
The force = +3 + 2k acts at the point (1, 1, 1). Find the torque of the force about
(a)
(b)
the point (2, -1, 5). Careful about the direction of ŕ between the two points.
the line = 21-+5k+ (i-+2k)t. Note that the line goes through the point (2, -1, 5).
Chapter 10 Solutions
EBK PHYSICS FOR SCIENTISTS & ENGINEERS
Ch. 10.1 - In Example 103, we found that the carousel, after...Ch. 10.4 - Two forces (FB = 20 N and FA = 30 N) are applied...Ch. 10.7 - In Figs. 1020f and g, the moments of inertia for a...Ch. 10.8 - Estimate the energy stored in the rotational...Ch. 10.9 - Return to the Chapter-Opening Question, p. 248,...Ch. 10.9 - Find the acceleration a of a yo-yo whose spindle...Ch. 10 - Prob. 1QCh. 10 - Suppose a disk rotates at constant angular...Ch. 10 - Could a nonrigid object be described by a single...Ch. 10 - Prob. 4Q
Ch. 10 - Prob. 5QCh. 10 - Prob. 6QCh. 10 - Can a small force ever exert a greater torque than...Ch. 10 - Why is it more difficult to do a sit-up with your...Ch. 10 - If the net force on a system is zero, is the net...Ch. 10 - Mammals that depend on being able to run fast have...Ch. 10 - Prob. 11QCh. 10 - Prob. 12QCh. 10 - Prob. 13QCh. 10 - Prob. 14QCh. 10 - Two inclines have the same height but make...Ch. 10 - Two spheres look identical and have the same mass....Ch. 10 - A sphere and a cylinder have the same radius and...Ch. 10 - Two solid spheres simultaneously start rolling...Ch. 10 - Prob. 1MCQCh. 10 - Prob. 2MCQCh. 10 - Prob. 3MCQCh. 10 - Prob. 4MCQCh. 10 - Prob. 6MCQCh. 10 - Prob. 7MCQCh. 10 - Prob. 8MCQCh. 10 - Prob. 9MCQCh. 10 - Prob. 10MCQCh. 10 - Prob. 11MCQCh. 10 - Prob. 12MCQCh. 10 - Prob. 14MCQCh. 10 - (I) Express the following angles in radians: (a)...Ch. 10 - Prob. 2PCh. 10 - Prob. 3PCh. 10 - (I) The blades in a blender rotate at a rate of...Ch. 10 - Prob. 5PCh. 10 - Prob. 6PCh. 10 - Prob. 7PCh. 10 - Prob. 8PCh. 10 - Prob. 9PCh. 10 - (II) A rotating merry-go-round makes one complete...Ch. 10 - Prob. 11PCh. 10 - Prob. 12PCh. 10 - (II) Calculate the angular velocity of the Earth...Ch. 10 - Prob. 14PCh. 10 - Prob. 15PCh. 10 - Prob. 16PCh. 10 - (II) A turntable of radius R1 is turned by a...Ch. 10 - Prob. 18PCh. 10 - (I) A centrifuge accelerates uniformly front rest...Ch. 10 - Prob. 20PCh. 10 - Prob. 21PCh. 10 - Prob. 22PCh. 10 - Prob. 23PCh. 10 - Prob. 24PCh. 10 - Prob. 25PCh. 10 - Prob. 26PCh. 10 - Prob. 27PCh. 10 - (II) Two blocks, each of mass m, are attached to...Ch. 10 - Prob. 29PCh. 10 - Prob. 30PCh. 10 - Prob. 31PCh. 10 - Prob. 32PCh. 10 - Prob. 33PCh. 10 - (I) Estimate the moment of inertia of a bicycle...Ch. 10 - Prob. 35PCh. 10 - (II) An oxygen molecule consists of two oxygen...Ch. 10 - Prob. 37PCh. 10 - (II) The forearm in Fig. 1052 accelerates a 3.6-kg...Ch. 10 - (II) Assume that a 1.00-kg ball is thrown solely...Ch. 10 - Prob. 40PCh. 10 - Prob. 41PCh. 10 - Prob. 42PCh. 10 - Prob. 43PCh. 10 - (II) A dad pushes tangentially on a small...Ch. 10 - Prob. 45PCh. 10 - Prob. 46PCh. 10 - Prob. 47PCh. 10 - Prob. 48PCh. 10 - (II) When discussing moments of inertia,...Ch. 10 - (II) Two blocks are connected by a light string...Ch. 10 - Prob. 51PCh. 10 - (III) A hammer thrower accelerates the hammer...Ch. 10 - (I) Use the parallel-axis theorem to show that the...Ch. 10 - (II) Determine the moment of inertia of a 19-kg...Ch. 10 - Prob. 55PCh. 10 - Prob. 56PCh. 10 - Prob. 57PCh. 10 - Prob. 58PCh. 10 - Prob. 61PCh. 10 - Prob. 62PCh. 10 - (I) Estimate the kinetic energy of the Earth with...Ch. 10 - (II) A rotating uniform cylindrical platform of...Ch. 10 - Prob. 65PCh. 10 - (II) A Uniform thin rod of length l and mass M is...Ch. 10 - Prob. 67PCh. 10 - (III) A 2.30-m-long pole is balanced vertically on...Ch. 10 - Prob. 69PCh. 10 - (I) A bowling ball of mass 7.3kg and radius 9.0 cm...Ch. 10 - Prob. 71PCh. 10 - (II) A narrow but solid spool of thread has radius...Ch. 10 - (II) A solid rubber ball rests on the floor of a...Ch. 10 - Prob. 74PCh. 10 - Prob. 75PCh. 10 - (II) A ball of radius r0 rolls on the inside of a...Ch. 10 - (III) A small sphere of radius r0 = 1.5 cm rolls...Ch. 10 - (III) A wheel with rotational inertia I=12MR2...Ch. 10 - (III) The 1100-kg mass of a car includes four...Ch. 10 - (I) A rolling hall slows down because the normal...Ch. 10 - Prob. 81GPCh. 10 - On a 12.0-cm-diameter audio compact disc (CD),...Ch. 10 - (a) A yo-yo is made of two solid cylindrical...Ch. 10 - Prob. 84GPCh. 10 - Prob. 85GPCh. 10 - A large spool of rope rolls on the ground with the...Ch. 10 - Bicycle gears: (a) How is the angular velocity R...Ch. 10 - Prob. 88GPCh. 10 - Figure 1065 illustrates an H2O molecule. The O H...Ch. 10 - Prob. 90GPCh. 10 - Prob. 91GPCh. 10 - Prob. 92GPCh. 10 - Prob. 93GPCh. 10 - Prob. 94GPCh. 10 - Prob. 96GPCh. 10 - A marble of mass m and radius r rolls along the...Ch. 10 - The density (mass per unit length) of a thin rod...Ch. 10 - If a billiard ball is hit in just the right way by...Ch. 10 - Prob. 100GPCh. 10 - When bicycle and motorcycle riders pop a wheelie,...Ch. 10 - A crucial part of a piece of machinery starts as a...Ch. 10 - Prob. 103GPCh. 10 - Prob. 104GPCh. 10 - Prob. 105GPCh. 10 - A thin uniform stick of mass M and length l is...Ch. 10 - Prob. 107GP
Additional Science Textbook Solutions
Find more solutions based on key concepts
If all of Earths nitrogen-fixing prokaryotes were to die suddenly, what would happen to the concentration of ni...
Biology: Life on Earth with Physiology (11th Edition)
47. Balance each chemical equation.
a.
b.
c.
d.
Introductory Chemistry (6th Edition)
SCIENTIFIC INQUIRY DRAW IT As a consequence of size alone, larger organisms tend to have larger brains than sm...
Campbell Biology (11th Edition)
Fibrous connective tissue consists of ground substance and fibers that provide strength, support, and flexibili...
Human Biology: Concepts and Current Issues (8th Edition)
Thiols such as ethanethiol and propanethiol can be used to reduce vitamin K epoxide to vitamin KH2, but they re...
Organic Chemistry (8th Edition)
4. Three groups of nonvascular plants are _______, ______, and _______. Three groups of seedless vascular plant...
Biology: Life on Earth (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 5 Find the total work done by forces A and B if the object undergoes the displacement C. Hint: Can you add the two forces first?arrow_forward1 F2 F₁ -F₁ F6 F₂ S A Work done on the particle as it moves through the displacement is positive. True False by the force Farrow_forwardA student measuring the wavelength produced by a vapour lamp directed the lightthrough two slits with a separation of 0.20 mm. An interference pattern was created on the screen,3.00 m away. The student found that the distance between the first and the eighth consecutive darklines was 8.0 cm. Draw a quick picture of the setup. What was the wavelength of the light emittedby the vapour lamp?arrow_forward
- A ball is tied to one end of a string. The other end of the string is fixed. The ball is set in motion around a vertical circle without friction. At the top of the circle, the ball has a speed of ; = √√ Rg, as shown in the figure. At what angle should the string be cut so that the ball will travel through the center of the circle? The path after string is cut Rarrow_forward(a) A luggage carousel at an airport has the form of a section of a large cone, steadily rotating about its vertical axis. Its metallic surface slopes downward toward the outside, making an angle of 24.5° with the horizontal. A 30.0-kg piece of luggage is placed on the carousel, 7.46 m from the axis of rotation. The travel bag goes around once in 37.5 s. Calculate the magnitude of the force of static friction between the bag and the carousel. Your response differs significantly from the correct answer. Rework your solution from the beginning and check each step carefully. N (b) The drive motor is shifted to turn the carousel at a higher constant rate of rotation, and the piece of luggage is bumped to a position 7.94 m from the axis of rotation. The bag is on the verge of slipping as it goes around once every 30.5 s. Calculate the coefficient of static friction between the bag and the carousel. Your response differs significantly from the correct answer. Rework your solution from the…arrow_forward(a) Imagine that a space probe could be fired as a projectile from the Earth's surface with an initial speed of 5.78 x 104 m/s relative to the Sun. What would its speed be when it is very far from the Earth (in m/s)? Ignore atmospheric friction, the effects of other planets, and the rotation of the Earth. (Consider the mass of the Sun in your calculations.) Your response is within 10% of the correct value. This may be due to roundoff error, or you could have a mistake in your calculation. Carry out all intermediate results to at least four-digit accuracy to minimize roundoff error. m/s (b) What If? The speed provided in part (a) is very difficult to achieve technologically. Often, Jupiter is used as a "gravitational slingshot" to increase the speed of a probe to the escape speed from the solar system, which is 1.85 x 104 m/s from a point on Jupiter's orbit around the Sun (if Jupiter is not nearby). If the probe is launched from the Earth's surface at a speed of 4.10 x 10 m/s relative…arrow_forward
- As shown in the figure, a roller-coaster track includes a circular loop of radius R in a vertical plane. A car of mass m is released from rest at a height h above the bottom of the circular section and then moves freely along the track with negligible energy loss due to friction. i (a) First suppose the car barely makes it around the loop; at the top of the loop, the riders are upside down and feel weightless. Find the required height h of the release point above the bottom of the loop. (Use any variable or symbol stated above along with the following as necessary: g.) h = (b) If the car is released at some point above the minimum required height, determine the amount by which the normal force on the car at the bottom of the loop exceeds the normal force on the car at the top of the loop. (Consider the moments when the car reaches the top and when it reaches the bottom again. Use any variable or symbol stated above along with the following as necessary: g.) NB - NT = The normal force…arrow_forwardOne of the more challenging elements in pairs figure skating competition is the "death spiral" (see the figure below), in which the female figure skater, balanced on one skate, is spun in a circle by the male skater. i The axis of rotation of the pair is vertical and through the toe of the skate on the male skater's leg that is bent backward, the toe being planted into the ice. During the one-armed maneuver first developed in the 1940s, the outstretched arm of the male skater must apply a large force to support a significant fraction of the female skater's weight and also to provide her centripetal acceleration. This force represents a danger to the structure of the wrist of the male skater. (a) Modeling the female skater, of mass 47.0 kg, as a particle, and assuming that the combined length of the two outstretched arms is 129 cm and that arms make an angle of 45.0° with the horizontal, what is the magnitude of the force (in N) exerted by the male skater's wrist if each turn is…arrow_forwardOne popular design of a household juice machine is a conical, perforated stainless steel basket 3.30 cm high with a closed bottom of diameter 8.00 cm and open top of diameter 14.40 cm that spins at 16000 revolutions per minute about a vertical axis. Solid pieces of fruit are chopped into granules by cutters at the bottom of the spinning cone. Then the fruit granules rapidly make their way to the sloping surface where the juice is extracted to the outside of the cone through the mesh perforations. The dry pulp spirals upward along the slope to be ejected from the top of the cone. The juice is collected in an enclosure immediately surrounding the sloped surface of the cone. Pulp Motor Spinning basket Juice spout (a) What centripetal acceleration does a bit of fruit experience when it is spinning with the basket at a point midway between the top and bottom? m/s² ---Direction--- (b) Observe that the weight of the fruit is a negligible force. What is the normal force on 2.00 g of fruit at…arrow_forward
- A satellite is in a circular orbit around the Earth at an altitude of 3.88 × 106 m. (a) Find the period of the orbit. (Hint: Modify Kepler's third law so it is suitable for objects orbiting the Earth rather than the Sun. The radius of the Earth is 6.38 × 106 m, and the mass of the Earth is 5.98 x 1024 kg.) h (b) Find the speed of the satellite. km/s (c) Find the acceleration of the satellite. m/s² toward the center of the eartharrow_forwardShown below is a waterslide constructed in the late 1800's. This slide was unique for its time due to the fact that a large number of small wheels along its length made friction negligible. Riders rode a small sled down the chute which ended with a horizontal section that caused the sled and rider to skim across the water much like a flat pebble. The chute was 9.76 m high at the top and 54.3 m long. Consider a rider and sled with a combined mass of 81.0 kg. They are pushed off the top of the slide from point A with a speed of 2.90 m/s, and they skim horizontally across the water a distance of 50 m before coming to rest. 9.76 m Engraving from Scientific American, July 1888 A (a) 20.0 m/ -54.3 m- 50.0 m (b) (a) Find the speed (in m/s) of the sled and rider at point C. 14.14 m/s (b) Model the force of water friction as a constant retarding force acting on a particle. Find the magnitude (in N) of the friction force the water exerts on the sled. 162.2 N (c) Find the magnitude (in N) of the…arrow_forwardA small object with mass 3.60 kg moves counterclockwise with constant angular speed 1.40 rad/s in a circle of radius 2.55 m centered at the origin. It starts at the point with position vector 2.551 m. Then it undergoes an angular displacement of 9.15 rad. (a) What is its new position vector? m (b) In what quadrant is the object located and what angle does its position vector make with the positive x-axis? ---Select--- ✓ at (c) What is its velocity? m/s (d) In what direction is it moving? (Give a negative angle.) ° from the +x direction. (e) What is its acceleration? m/s² (f) What total force is exerted on the object? Narrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College
Moment of Inertia; Author: Physics with Professor Matt Anderson;https://www.youtube.com/watch?v=ZrGhUTeIlWs;License: Standard Youtube License