
Concept explainers
a)
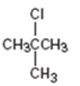
Interpretation:
How to prepare tert-butyl chloride from tert-butylalcohol is to be stated.
Concept introduction:
tert-Alcohols when treated with HCl, HBr or HI in ether at 0oC give the corresponding tert-
To state:
How to prepare tert-butyl chloride from tert-butylalcohol.
b)
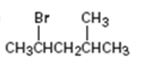
Interpretation:
How to .prepare 2-bromo-4-methylpentane from the corresponding alcohol is to be stated.
Concept introduction:
A secondary alkyl bromide is required. Secondary alkyl halides can be prepared by treating the alcohol required with PBr3 in ether solution.
To state:
How to prepare 2-bromo-4-methylpentane from the corresponding alcohol.
c)
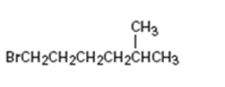
Interpretation:
How to .prepare 1-bromo-5-methylhexane from the corresponding alcohol is to be stated.
Concept introduction:
A primary alkyl bromide is required. Primary alkyl halides can be prepared by treating the alcohol required with PBr3 in ether solution.
To state:
How to prepare1-bromo-5-methylhexane from the corresponding alcohol.
d)
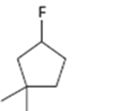
Interpretation:
How to .prepare 1-fluoro-3,3-dimethylcyclopentane from the corresponding alcohol is to be stated.
Concept introduction:
A secondary alkyl fluoride is required. Secondary alkyl fluorides can be prepared by treating the alcohol required with diethylaminosulphur trifluoride and HF in pyridine.
To state:
How to prepare1-fluoro-3,3-dimethylcyclopentane from the corresponding alcohol.
Trending nowThis is a popular solution!

Chapter 10 Solutions
Study Guide with Student Solutions Manual for McMurry's Organic Chemistry, 9th
- What is the name of the following compound? SiMe3arrow_forwardK Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing 1. NaNH2 2. PhCH2Br 4 57°F Sunny Q Searcharrow_forward7 Draw the starting alkyl bromide that would produce this alkyne under these conditions. F Drawing 1. NaNH2, A 2. H3O+ £ 4 Temps to rise Tomorrow Q Search H2arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

