
Chemistry in Context
8th Edition
ISBN: 9780073522975
Author: American Chemical Society
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10.3, Problem 10.9YT
Skill Building Ester Formation
Draw structural formulas for the esters that form when these alcohol and acid pairs react.
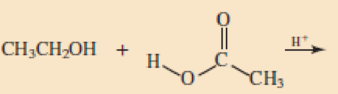
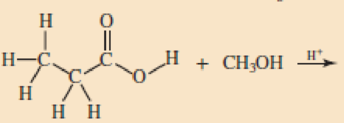
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the missing reactant R in this organic reaction?
N
N
དལ་ད་་
+ R
• Draw the structure of R in the drawing area below.
• Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer.
Click and drag to start drawing a
structure.
ㄖˋ
Draw the condensed structure of 4-hydroxy-3-methylbutanal.
Click anywhere to draw the first
atom of your structure.
Using the bond energy values, calculate the energy that must be supplied or is released upon the polymerization of 755 monomers. If energy must be supplied, provide a positive number; if energy is released, provide a negative number. Hint: Avogadro’s number is 6.02 × 1023.
Chapter 10 Solutions
Chemistry in Context
Ch. 10.2 - Skill Building Checking on Carbon a. Examine the...Ch. 10.2 - Prob. 10.7YTCh. 10.3 - Skill Building Ester Formation Draw structural...Ch. 10.3 - Prob. 10.10YTCh. 10.4 - Prob. 10.11CTCh. 10.4 - You Decide Supersize My Aspirin A friend who...Ch. 10.5 - Modern methods of drug discovery involve...Ch. 10.5 - Make two lists of drugs for each of the two...Ch. 10.5 - See for yourself the shapes of drug molecules by...Ch. 10.6 - Prob. 10.17YT
Ch. 10.6 - Prob. 10.18CTCh. 10.10 - Prob. 10.24CTCh. 10.10 - Prob. 10.25CTCh. 10.10 - Prob. 10.26YTCh. 10 - Prob. 10.1CTCh. 10 - Prob. 1QCh. 10 - Prob. 2QCh. 10 - Prob. 3QCh. 10 - Write the structural formula and line-angle...Ch. 10 - Prob. 5QCh. 10 - Prob. 6QCh. 10 - Prob. 7QCh. 10 - Prob. 8QCh. 10 - Prob. 9QCh. 10 - Prob. 10QCh. 10 - Prob. 11QCh. 10 - Prob. 12QCh. 10 - Prob. 13QCh. 10 - Prob. 14QCh. 10 - Prob. 15QCh. 10 - Prob. 16QCh. 10 - Prob. 17QCh. 10 - Prob. 18QCh. 10 - Prob. 19QCh. 10 - Prob. 20QCh. 10 - Prob. 21QCh. 10 - Sulfanilamide is the simplest sulfa drug, a type...Ch. 10 - Prob. 23QCh. 10 - Prob. 24QCh. 10 - Prob. 25QCh. 10 - Prob. 26QCh. 10 - Molecules as diverse as cholesterol, sex hormones,...Ch. 10 - The text states that some racemic mixtures contain...Ch. 10 - Draw structural formulas for each of these...Ch. 10 - Prob. 30QCh. 10 - In Your Turn 12.12, you were asked to draw...Ch. 10 - Prob. 32QCh. 10 - Prob. 33QCh. 10 - Prob. 34QCh. 10 - Prob. 35QCh. 10 - Prob. 36QCh. 10 - Prob. 37QCh. 10 - Prob. 38QCh. 10 - Prob. 39QCh. 10 - Prob. 40QCh. 10 - Prob. 41QCh. 10 - Prob. 42QCh. 10 - Dorothy Crowfoot Hodgkin first determined the...Ch. 10 - Prob. 46QCh. 10 - Prob. 47QCh. 10 - Prob. 49QCh. 10 - Prob. 51QCh. 10 - Prob. 52QCh. 10 - Prob. 54QCh. 10 - Prob. 55QCh. 10 - Prob. 56QCh. 10 - Prob. 57QCh. 10 - Prob. 58QCh. 10 - Prob. 59Q
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- -AG|F=2E|V 3. Before proceeding with this problem you may want to glance at p. 466 of your textbook where various oxo-phosphorus derivatives and their oxidation states are summarized. Shown below are Latimer diagrams for phosphorus at pH values at 0 and 14: Acidic solution -0.93 +0.38 -0.51 -0.06 H3PO4 →H4P206 H3PO3 H3PO2 → P→ PH3 -0.28 -0.50 → -0.50 Basic solution 3-1.12 -1.57 -2.05 -0.89 PO HPO →→H2PO2 P PH3 -1.73 a) Under acidic conditions, H3PO4 can be reduced into H3PO3 directly (-0.28V), or via the formation and reduction of H4P2O6 (-0.93/+0.38V). Calculate the values of AG's for both processes; comment. (3 points) 0.5 PH, 0.0 -0.5- 2 3 9 3 -1.5 -2.0 Pa H,PO H,PO H,PO -3 -1 0 2 4 Oxidation state, N 2 b) Frost diagram for phosphorus under acidic conditions is shown. Identify possible disproportionation and comproportionation processes; write out chemical equations describing them. (2 points) c) Elemental phosphorus tends to disproportionate under basic conditions. Use data in…arrow_forwardThese two reactions appear to start with the same starting materials but result in different products. How do the chemicals know which product to form? Are both products formed, or is there some information missing that will direct them a particular way?arrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 3 1 2 2. n-BuLi • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Priva ×arrow_forward
- Predict the products of this organic reaction: Explanation Check IN NaBH3CN H+ ? Click and drag to start drawing a structure. D 5 C +arrow_forwardPredict the products of this organic reaction: H3O+ + ? • Draw all the reasonable products in the drawing area below. If there are no products, because no reaction will occur, check the box under the drawing area. • Include both major and minor products, if some of the products will be more common than others. • Be sure to use wedge and dash bonds if you need to distinguish between enantiomers. No reaction. Click and drag to start drawing a structure. dmarrow_forwardIarrow_forward
- Draw the anti-Markovnikov product of the hydration of this alkene. this problem. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for esc esc ☐ Explanation Check F1 1 2 F2 # 3 F3 + $ 14 × 1. BH THE BH3 2. H O NaOH '2 2' Click and drag to start drawing a structure. F4 Q W E R A S D % 905 LL F5 F6 F7 © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility < & 6 7 27 8 T Y U G H I F8 F9 F10 F11 F12 9 0 J K L P + // command option Z X C V B N M H H rol option commandarrow_forwardAG/F-2° V 3. Before proceeding with this problem you may want to glance at p. 466 of your textbook where various oxo-phosphorus derivatives and their oxidation states are summarized. Shown below are Latimer diagrams for phosphorus at pH values at 0 and 14: -0.93 +0.38 -0.50 -0.51 -0.06 H3PO4 →H4P206 →H3PO3 →→H3PO₂ → P → PH3 Acidic solution Basic solution -0.28 -0.50 3--1.12 -1.57 -2.05 -0.89 PO HPO H₂PO₂ →P → PH3 -1.73 a) Under acidic conditions, H3PO4 can be reduced into H3PO3 directly (-0.28V), or via the formation and reduction of H4P206 (-0.93/+0.38V). Calculate the values of AG's for both processes; comment. (3 points) 0.5 PH P 0.0 -0.5 -1.0- -1.5- -2.0 H.PO, -2.3+ -3 -2 -1 1 2 3 2 H,PO, b) Frost diagram for phosphorus under acidic conditions is shown. Identify possible disproportionation and comproportionation processes; write out chemical equations describing them. (2 points) H,PO 4 S Oxidation stale, Narrow_forward4. For the following complexes, draw the structures and give a d-electron count of the metal: a) Tris(acetylacetonato)iron(III) b) Hexabromoplatinate(2-) c) Potassium diamminetetrabromocobaltate(III) (6 points)arrow_forward
- 2. Calculate the overall formation constant for [Fe(CN)6]³, given that the overall formation constant for [Fe(CN)6] 4 is ~1032, and that: Fe3+ (aq) + e = Fe²+ (aq) E° = +0.77 V [Fe(CN)6]³ (aq) + e¯ = [Fe(CN)6] (aq) E° = +0.36 V (4 points)arrow_forward5. Consider the compounds shown below as ligands in coordination chemistry and identify their denticity; comment on their ability to form chelate complexes. (6 points) N N A B N N N IN N Carrow_forward1. Use standard reduction potentials to rationalize quantitatively why: (6 points) (a) Al liberates H2 from dilute HCl, but Ag does not; (b) Cl2 liberates Br2 from aqueous KBr solution, but does not liberate C12 from aqueous KCl solution; c) a method of growing Ag crystals is to immerse a zinc foil in an aqueous solution of AgNO3.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Quantum Mechanics - Part 1: Crash Course Physics #43; Author: CrashCourse;https://www.youtube.com/watch?v=7kb1VT0J3DE;License: Standard YouTube License, CC-BY