
Concept explainers
(a)
Interpretation:
Structural formula of the ester formed by the reaction between acetic acid and n-propanol has to be drawn.
Concept introduction:
Ester: One
Ester formation: Esters are prepared by the reaction of a
Here, the
In chemistry, structure is the arrangement of
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
(a)
Explanation of Solution
Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule.
Here, the carboxylic acid is,
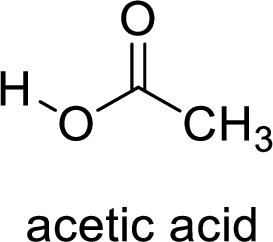
Alcohol is,
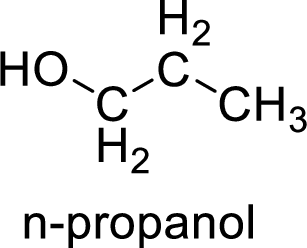
Here, the
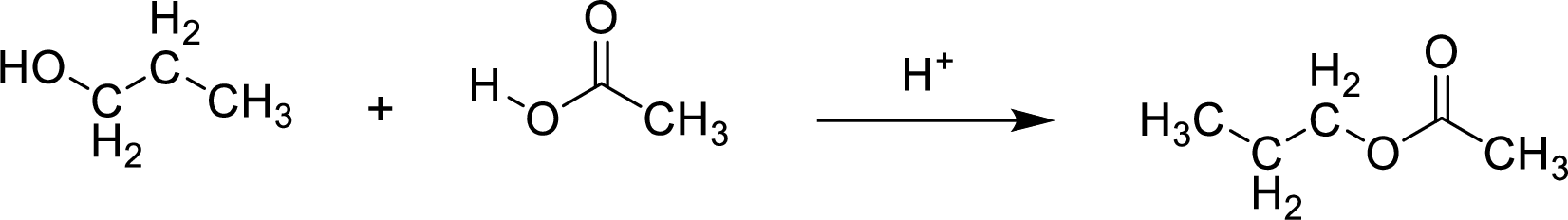
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
Therefore,
The structural formulas for the ester formed is,
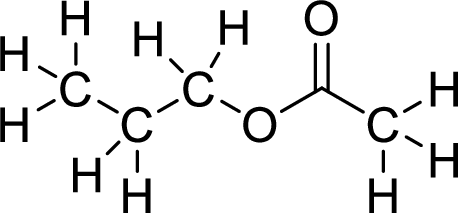
(b)
Interpretation:
Structural formula of the ester formed by the reaction between acetic acid and iso-propanol has to be drawn.
Concept introduction:
Ester: One
Ester formation: Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule. The addition of a strong acid such as
Here, the
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
(b)
Explanation of Solution
Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule.
Here, the carboxylic acid is,
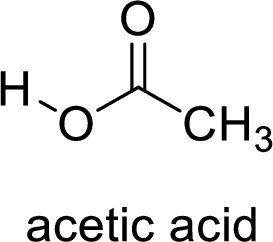
Alcohol is,
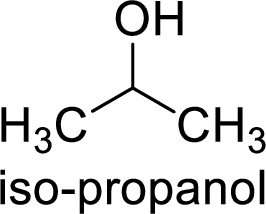
Here, the
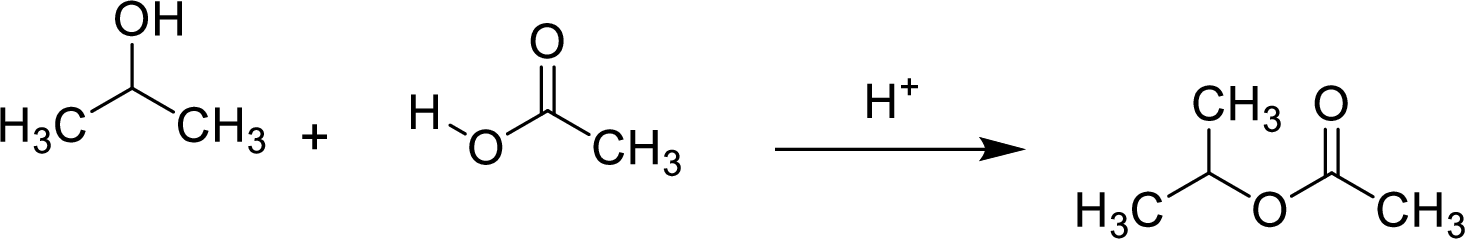
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
Therefore,
The structural formulas for the ester formed is,
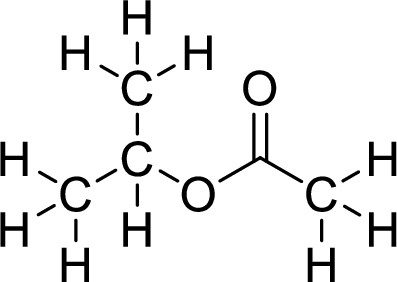
(c)
Interpretation:
Structural formula of the ester formed by the reaction between acetic acid and iso-propanol has to be drawn.
Concept introduction:
Ester: One
Ester formation: Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule. The addition of a strong acid such as
Here, the
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds
- ✓ H atoms are shown
(c)
Explanation of Solution
Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule.
Here, the carboxylic acid is,
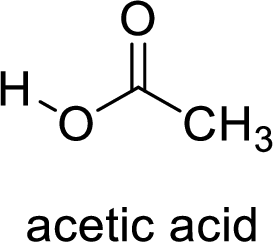
Alcohol is,
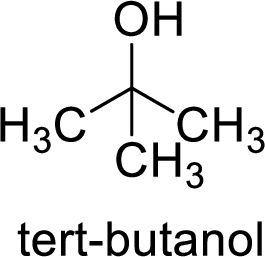
Here, the
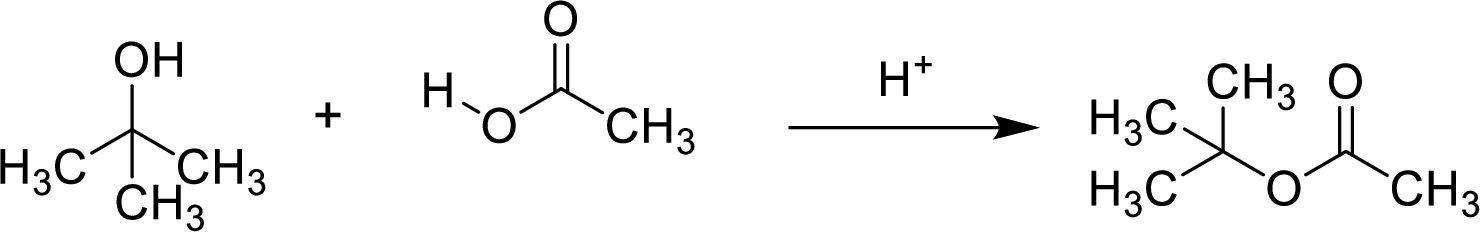
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
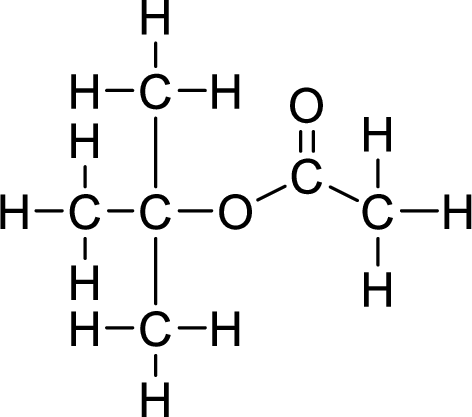
Therefore,
The structural formulas for the ester formed is,
Want to see more full solutions like this?
Chapter 10 Solutions
Chemistry in Context
- Draw the stepwise mechanism for the reactionsarrow_forwardPart I. a) Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone b) Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone (3,3-dimethyl-2-butanone) and 2, 3-dimethyl - 1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forward3. The explosive decomposition of 2 mole of TNT (2,4,6-trinitrotoluene) is shown below: Assume the C(s) is soot-basically atomic carbon (although it isn't actually atomic carbon in real life). 2 CH3 H NO2 NO2 3N2 (g)+7CO (g) + 5H₂O (g) + 7C (s) H a. Use bond dissociation energies to calculate how much AU is for this reaction in kJ/mol.arrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: Pinacol (2,3-dimethyl, 1-3-butanediol) on treatment w/ acid gives a mixture of pina colone and (3,3-dimethyl-2-butanone) 2,3-dimethyl-1,3-butadiene. Give reasonable mechanism the formation of the products Forarrow_forwardShow the mechanism for these reactionsarrow_forwardDraw the stepwise mechanismarrow_forward
- Draw a structural formula of the principal product formed when benzonitrile is treated with each reagent. (a) H₂O (one equivalent), H₂SO₄, heat (b) H₂O (excess), H₂SO₄, heat (c) NaOH, H₂O, heat (d) LiAlH4, then H₂Oarrow_forwardDraw the stepwise mechanism for the reactionsarrow_forwardDraw stepwise mechanismarrow_forward
- Part I. Draw reaction mechanism for the transformations of benzophenone to benzopinacol to benzopinaco lone and answer the ff: a) Give the major reason for the exposure of benzophenone al isopropyl alcohol (w/acid) to direct sunlight of pina colone Mechanism For b) Pinacol (2,3-dimethy 1, 1-3-butanediol) on treatment w/ acid gives a mixture (3,3-dimethyl-2-butanone) and 2, 3-dimethyl-1,3-butadiene. Give reasonable the formation of the productsarrow_forwardwhat are the Iupac names for each structurearrow_forwardWhat are the IUPAC Names of all the compounds in the picture?arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





