
EBK WEBASSIGN FOR ZUMDAHL'S CHEMICAL PR
8th Edition
ISBN: 9780357119099
Author: ZUMDAHL
Publisher: VST
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 10, Problem 82E
Consider the following diagram of free energy (G) versusfraction of A reacted in terms of moles for the reaction
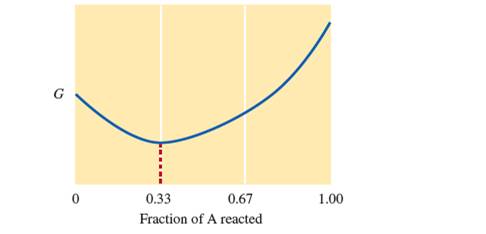
Before any A has reacted,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
EBK WEBASSIGN FOR ZUMDAHL'S CHEMICAL PR
Ch. 10 - For the process A(l)A(g) , which direction is...Ch. 10 - Prob. 2DQCh. 10 - Prob. 3DQCh. 10 - Prob. 4DQCh. 10 - Prob. 5DQCh. 10 - Prob. 6DQCh. 10 - Predict the sign of S for each of the following...Ch. 10 - Prob. 8DQCh. 10 - Prob. 9DQCh. 10 - At 1 atm, liquid water is heated above 100°C. For...
Ch. 10 - Prob. 11DQCh. 10 - Prob. 12ECh. 10 - Prob. 13ECh. 10 - Prob. 14ECh. 10 - Consider the following energy levels, each capable...Ch. 10 - Prob. 16ECh. 10 - Prob. 17ECh. 10 - Which of the following involve an increase in the...Ch. 10 - Prob. 19ECh. 10 - Choose the substance with the larger positional...Ch. 10 - In the roll of two dice, what total number is the...Ch. 10 - Entropy can be calculated by a relationship...Ch. 10 - Calculate the energy required to change the...Ch. 10 - For nitrogen gas the values of CvandCp at 25°Care...Ch. 10 - Consider a rigid, insulated box containing 0.400...Ch. 10 - One mole of an ideal gas is contained in a...Ch. 10 - One mole of an ideal gas with a volume of 1.0 L...Ch. 10 - A cylinder with an initial volume of 10.0 L is...Ch. 10 - The molar heat capacities for carbon dioxide at...Ch. 10 - The molar entropy of helium gas at 25°C and 1.00...Ch. 10 - Consider the process A(l)A(g)75C155C which is...Ch. 10 - A sample of ice weighing 18.02 g, initially at...Ch. 10 - Calculate the entropy change for a process in...Ch. 10 - Calculate the change in entropy that occurs...Ch. 10 - The synthesis of glucose directly from CO2andH2O...Ch. 10 - A green plant synthesizes glucose by...Ch. 10 - Entropy has been described as “time’s arrow.”...Ch. 10 - For a gas phase reaction, what do you concentrate...Ch. 10 - What determines Ssurr for a process? To calculate...Ch. 10 - Predict the sign of Ssurr for the following...Ch. 10 - Calculate Ssurr for the following reactions at...Ch. 10 - For each of the following pairs of substances,...Ch. 10 - Predict the sign of S for each of the following...Ch. 10 - Prob. 44ECh. 10 - Prob. 45ECh. 10 - For the reaction CS2(g)+3O2(g)CO2(g)+2SO2(g) S is...Ch. 10 - For the reaction C2H2(g)+4F2(g)2CF4(g)+H2(g) S is...Ch. 10 - Ethanethiol ( C2H5SH ; also called ethyl...Ch. 10 - For mercury at 1 atm, the enthalpy of vaporization...Ch. 10 - The enthalpy of vaporization of ethanol is 38.7...Ch. 10 - For ammonia (NH3) the enthalpy of fusion is 5.65...Ch. 10 - It is quite common for a solid to change from one...Ch. 10 - As O2(l) is cooled at 1 atm, it freezes at 54.5 K...Ch. 10 - Prob. 54ECh. 10 - The value of G for the reaction...Ch. 10 - Of the functions H,S,andG , which dependsmost...Ch. 10 - For the reaction at 29° K, 2NO2(g)N2O4(g) the...Ch. 10 - Consider the reaction...Ch. 10 - Consider the reaction 2POCl3(g)2PCl3(g)+O2(g) a....Ch. 10 - Consider two reactions for the production of...Ch. 10 - Prob. 61ECh. 10 - Prob. 62ECh. 10 - When most biological enzymes are heated, they...Ch. 10 - For the reaction 2O(g)O2(g) a. predict the signs...Ch. 10 - Hydrogen cyanide is produced industrially by the...Ch. 10 - A reaction at constant T and P is spontaneous as...Ch. 10 - G predicts spontaneity for a reaction at constant...Ch. 10 - Using thermodynamic data from Appendix 4,...Ch. 10 - Prob. 69ECh. 10 - Using data from Appendix 4, calculate G for...Ch. 10 - Prob. 71ECh. 10 - One of the reactions that destroys ozone in the...Ch. 10 - Hydrogen sulfide can be removed from natural gas...Ch. 10 - Consider the autoionization of water at 25°C:...Ch. 10 - How can one estimate the value of K at...Ch. 10 - The standard free energies of formation and the...Ch. 10 - Consider the reaction...Ch. 10 - Prob. 78ECh. 10 - Consider the following reaction at 800. K:...Ch. 10 - Consider the following reaction at 298 K:...Ch. 10 - For the reaction A(g)+2B(g)C(g) the initial...Ch. 10 - Consider the following diagram of free energy (G)...Ch. 10 - Calculate G for H2O(g)+12O2(g)H2O2(g) at600. K,...Ch. 10 - Cells use the hydrolysis of adenosine...Ch. 10 - Carbon monoxide is toxic because it bonds much...Ch. 10 - One reaction that occurs in human metabolism is...Ch. 10 - At 25.0°C, for the reaction 2NO2(g)N2O4(g) the...Ch. 10 - Consider the relationship ln(K)=HRT+SR The...Ch. 10 - a. Use the equation in Exercise 88 to determine H...Ch. 10 - The equilibrium constant K for the reaction...Ch. 10 - The equilibrium constant for a certain reaction...Ch. 10 - A sample of a monatomic ideal gas at 1.00 atm...Ch. 10 - A sample of 1.75 moles of H2(Cv=20.5JK-1mol-1) at...Ch. 10 - A 1.50-mole sample of an ideal gas is allowed to...Ch. 10 - Consider 1.00 mole of CO2(g) at 300. K and 5.00...Ch. 10 - Prob. 96ECh. 10 - A mixture of hydrogen gas and chlorine gas...Ch. 10 - When the environment is contaminated by a toxic...Ch. 10 - If you calculate a value for G for a reaction...Ch. 10 - Given the following illustration, what can be said...Ch. 10 - Some water is placed in a coffee cup calorimeter....Ch. 10 - Using Appendix 4 and the following data, determine...Ch. 10 - Prob. 103AECh. 10 - Human DNA contains almost twice as much...Ch. 10 - The enthalpy of vaporization of chloroform (CHCl3)...Ch. 10 - Two crystalline forms of white phosphorus are...Ch. 10 - Monochloroethane (C2H5Cl) can be produced by...Ch. 10 - Acrylonitrile is the starting material used in the...Ch. 10 - Prob. 109AECh. 10 - Many biochemical reactions that occur in cells...Ch. 10 - Consider the following reaction at 35°C:...Ch. 10 - Consider the reaction H2(g)+Br2(g)2HBr(g) where...Ch. 10 - At 1500 K the process I2(g)2I(g)10atm10atm is not...Ch. 10 - Using the following data, calculate the value of...Ch. 10 - Sodium chloride is added to water (at 25°C) until...Ch. 10 - Prob. 116AECh. 10 - Prob. 117AECh. 10 - The deciding factor on why HF is a weak acid and...Ch. 10 - Prob. 119AECh. 10 - Calculate the entropy change for the vaporization...Ch. 10 - The standard entropy values (S°) for...Ch. 10 - Calculate the values of S and G for each of the...Ch. 10 - Calculate the changes in free energy, enthalpy,...Ch. 10 - Consider the isothermal expansion of 1.00 mole of...Ch. 10 - A 1.00-mole sample of an ideal gas in a vessel...Ch. 10 - One mole of an ideal gas with a volume of 6.67 L...Ch. 10 - Which of the following reactions (or processes)...Ch. 10 - For rubidium Hvap=69.0kJ/mol at 686°C, its...Ch. 10 - Given the thermodynamic data below, calculate S...Ch. 10 - Consider the reaction: H2S(g)+SO2(g)3S(g)+2H2O(l)...Ch. 10 - The following reaction occurs in pure water:...Ch. 10 - Consider the dissociation of a weak acid HA...Ch. 10 - Consider the reaction: PCl3(g)+Cl2(g)PCl5(g) a....Ch. 10 - The equilibrium constant for a certain reaction...Ch. 10 - Consider a 2.00-mole sample of Ar at 2.00 atm...Ch. 10 - Prob. 136CPCh. 10 - One mole of an ideal gas undergoes an isothermal...Ch. 10 - At least some of what is in the following quoted...Ch. 10 - You have a 1.00-L sample of hot water (90.°C)...Ch. 10 - Consider two perfectly insulated vessels. Vessel 1...Ch. 10 - If wet silver carbonate is dried in a stream of...Ch. 10 - Consider a weak acid HX. If a 0.10 M solution of...Ch. 10 - Using data from Appendix 4, calculate H , G , and...Ch. 10 - One mole of a monatomic ideal gas (for which...Ch. 10 - Consider the system A(g)B(g) a. 25°C. a. Assuming...Ch. 10 - Liquid water at 25°C is introduced into an...Ch. 10 - Consider 1.00 mole of an ideal gas that is...Ch. 10 - Prob. 148CPCh. 10 - Consider the reaction 2CO(g)+O2(g)2CO2(g) a. Using...Ch. 10 - Prob. 150CPCh. 10 - Prob. 151CPCh. 10 - Consider the following Cp values for N2(g) :...Ch. 10 - Benzene (C6H6) has a melting point of 5.5°C and...Ch. 10 - Prob. 154MPCh. 10 - Prob. 155MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which contains greater entropy, a quantity of frozen benzene or the same quantity of liquid benzene at the same temperature? Explain in terms of the dispersal of energy in the substance.arrow_forwardUse the data in Appendix J to calculate rG andKPat 25 C for the reaction 2HBr(g)+Cl2(g)2HCl(g)+Br2() Comment on the connection between the sign of rG and the magnitude ofKP.arrow_forwardConsider the reaction of 1 mol H2(g) at 25C and 1 atm with 1 mol Br2(l) at the same temperature and pressure to produce gaseous HBr at these conditions. If this reaction is run in a controlled way to generate work, what is the maximum useful work that can be obtained? How much entropy is produced in this case?arrow_forward
- Consider the reaction of 2 mol H2(g) at 25C and 1 atm with 1 mol O2(g) at the same temperature and pressure to produce liquid water at these conditions. If this reaction is run in a controlled way to generate work, what is the maximum useful work that can be obtained? How much entropy is produced in this case?arrow_forwardThe combustion of acetylene, C2H2, is a spontaneous reaction given by the equation 2C2H2(g)+5O2(g)4CO2(g)+2H2O(l) As expected for a combustion, the reaction is exothermic. What is the sign of H? What do you expect for the sign of S? Explain the spontaneity of the reaction in terms of the enthalpy and entropy changes.arrow_forwardConsider the decomposition of red mercury(II) oxide under standard state conditions.. 2HgO(s,red)2Hg(l)+O2(g) (a) Is the decomposition spontaneous under standard state conditions? (b) Above what temperature does the reaction become spontaneous?arrow_forward
- Elemental boron, in the form of thin fibers, can be made by reducing a boron halide with H2. BCl3(g) + 3/2 H2(g) B(s) + 3HCl(g) Calculate H, S, and G at 25 C for this reaction. Is the reaction predicted to be product favored at equilibrium at 25 C? If so, is it enthalpy driven or entropy driven?arrow_forwardSilver carbonate, Ag2CO3, is a light yellow compound that decomposes when heated to give silver oxide and carbon dioxide: Ag2CO3(s)Ag2O(s)+CO2(g) A researcher measured the partial pressure of carbon dioxide over a sample of silver carbonate at 220C and found that it was 1.37 atm. Calculate the partial pressure of carbon dioxide at 25C. The standard enthalpies of formation of silver carbonate and silver oxide at 25C are 505.9 kJ/mol and 31.05 kJ/mol, respectively. Make any reasonable assumptions in your calculations. State the assumptions that you make, and note why you think they are reasonable.arrow_forwardThe free energy for a reaction decreases as temperature increases. Explain how this observation is used to determine the sign of either H or S.arrow_forward
- A crucial reaction for the production of synthetic fuels is the production of H2 by the reaction of coal with steam. The chemical reaction is C(s) + H2O(g) CO(g) + H2(g) (a) Calculate rG for this reaction at 25 C, assuming C(s) is graphite. (b) Calculate Kp for the reaction at 25 C. (c) Is the reaction predicted to be product-favored at equilibrium at 25 C? If not, at what temperature will it become so?arrow_forwardFor each of the following processes, identify the systemand the surroundings. Identify those processes that arespontaneous. For each spontaneous process, identify theconstraint that has been removed to enable the process to occur: Ammonium nitrate dissolves in water. Hydrogen and oxygen explode in a closed bomb. A rubber band is rapidly extended by a hangingweight. The gas in a chamber is slowly compressed by aweighted piston. A glass shatters on the floor.arrow_forwardUse S values to calculate the standard entropy change, rS0, for each of the following processes and comment on the sign of the change. (a) KOH(s) KOH(aq) (b) Na(g) Na(s) (c) Br2() Br2(g) (d) HCl(g) HCl(aq)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY