
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
4th Edition
ISBN: 9780134110684
Author: Randall D. Knight (Professor Emeritus)
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 29EAP
In FIGURE EX10.28, what is the maximum speed a 200 g particle could have at x = 2.0 m and never reach x = 6.0 m?
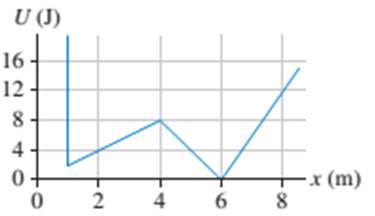
FIGURE EX10.28
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
10. Imagine you have a system in which you have 54 grams of ice. You can melt this
ice and then vaporize it all at 0 C. The melting and vaporization are done reversibly
into a balloon held at a pressure of 0.250 bar. Here are some facts about water you
may wish to know. The density of liquid water at 0 C is 1 g/cm³. The density of ice at 0
C is 0.917 g/cm³. The enthalpy of vaporization of liquid water is 2.496 kJ/gram and the
enthalpy of fusion of solid water is 333.55 J/gram.
Consider 1 mole of supercooled water at -10°C. Calculate the entropy change of the water when the
supercooled water freezes at -10°C and 1 atm.
Useful data:
Cp (ice) = 38 J mol-1 K-1
Cp (water) 75J mol −1
K
-1
Afus H (0°C) 6026 J mol −1
Assume Cp (ice) and Cp (water) to be independent of temperature.
The molar enthalpy of vaporization of benzene at its normal boiling point (80.09°C) is 30.72 kJ/mol.
Assuming that AvapH and AvapS stay constant at their values at 80.09°C, calculate the value of
AvapG at 75.0°C, 80.09°C, and 85.0°C.
Hint: Remember that the liquid and vapor phases will be in equilibrium at the normal boiling point.
Chapter 10 Solutions
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
Ch. 10 - Prob. 1CQCh. 10 - Can kinetic energy ever be negative? Can...Ch. 10 - Prob. 3CQCh. 10 - 4. The three balls in FIGURE Q1O.4, which have...Ch. 10 - Rank in order, from most to least, the elastic...Ch. 10 - 6. A spring is compressed 1.0 cm. How far must you...Ch. 10 - Prob. 7CQCh. 10 - A particle with the potential energy shown in...Ch. 10 - A compressed spring launches a block up an...Ch. 10 - 10. A process occurs in which a system’s potential...
Ch. 10 - A process occurs in which a system’s potential...Ch. 10 - FIGURE Q10.12 is the energy bar chart for a...Ch. 10 - Prob. 13CQCh. 10 - Object A is stationary while objects B and C are...Ch. 10 - Prob. 2EAPCh. 10 - 3. The lowest point in Death Valley is 85 m below...Ch. 10 - Prob. 4EAPCh. 10 - Prob. 5EAPCh. 10 - 6. What height does a frictionless playground...Ch. 10 - 7. A 55 kg skateboarder wants to just make it to...Ch. 10 - Prob. 8EAPCh. 10 - A pendulum is made by tying a 500 g ball to a...Ch. 10 - A 20 kg child is on a swing that hangs from...Ch. 10 - A 1500 kg car traveling at 10 m/s suddenly runs...Ch. 10 - Prob. 12EAPCh. 10 - A cannon tilted up at a 30° angle fires a cannon...Ch. 10 - In a hydroelectric dam, water falls 25 m and then...Ch. 10 - How far must you stretch a spring with k = 000 N/m...Ch. 10 - A stretched spring stores 2.0 J of energy. How...Ch. 10 - A student places her 500 g physics book on a...Ch. 10 - A block sliding along a horizontal frictionless...Ch. 10 - A 10 kg runaway grocery cart runs into a spring...Ch. 10 - As a 15,000 kg jet plane lands on an aircraft...Ch. 10 - The elastic energy stored in your tendons can...Ch. 10 - The spring in FIGURE EX10.22a is compressed by ?x....Ch. 10 - The spring in FIGURE EXIO.23a is compressed by ?x....Ch. 10 - FIGURE EX10.24 is the potential-energy diagram for...Ch. 10 - Prob. 25EAPCh. 10 - In FIGURE EX10.26, what is the maximum speed of a...Ch. 10 - Prob. 27EAPCh. 10 - FIGURE EX10.28 shows the potential energy of a 500...Ch. 10 - In FIGURE EX10.28, what is the maximum speed a 200...Ch. 10 - A system in which only one particle can move has...Ch. 10 - A system in which only one particle can move has...Ch. 10 - A particle moving along the y-axis is in a system...Ch. 10 - A particle moving along the x-axis is in a system...Ch. 10 - FIGURE EX10.34 shows the potential energy of a...Ch. 10 - A particle moves from A to D in FIGURE EX10.35...Ch. 10 - A force does work on a 50 g particle as the...Ch. 10 - A system loses 400 J of potential energy. In the...Ch. 10 - What is the final kinetic energy of the system for...Ch. 10 - How much work is done by the environment in the...Ch. 10 - A cable with 20.0 N tension pulls straight up on a...Ch. 10 - A very slippery ice cube slides in a vertical...Ch. 10 - A 50 g ice cube can slide up and down a...Ch. 10 - You have been hired to design a spring-launched...Ch. 10 - It’s been a great day of new, frictionless snow....Ch. 10 - Prob. 45EAPCh. 10 - A 1000 kg safe is 2.0 m above a heavy-duty spring...Ch. 10 - You have a ball of unknown mass, a spring with...Ch. 10 - Sam, whose mass is 75 kg, straps on his skis and...Ch. 10 - A horizontal spring with spring constant 100 N/m...Ch. 10 - Truck brakes can fail if they get too hot. In some...Ch. 10 - Prob. 51EAPCh. 10 - Use work and energy to find an expression for the...Ch. 10 - Prob. 53EAPCh. 10 - The spring shown in FIGURE 10.54 is compressed 50...Ch. 10 - Prob. 55EAPCh. 10 - Prob. 56EAPCh. 10 - A system has potential energy U(x) = x + sin ((2...Ch. 10 - Prob. 58EAPCh. 10 - Prob. 59EAPCh. 10 - Prob. 60EAPCh. 10 - The potential energy for a particle that can move...Ch. 10 - A particle that can move along the x-axis...Ch. 10 - An object moving in the xy-plane is subjected to...Ch. 10 - An object moving in the xy-plane is subjected to...Ch. 10 - Prob. 65EAPCh. 10 - In Problems 66 through 68 you are given the...Ch. 10 - Prob. 67EAPCh. 10 - Prob. 68EAPCh. 10 - A pendulum is formed from a small ball of mass m...Ch. 10 - Prob. 70EAPCh. 10 - Prob. 71EAPCh. 10 - Prob. 72EAPCh. 10 - The spring in FIGURE CP10.73 has a spring constant...Ch. 10 - A sled starts from rest at the top of the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- 3. The entropy of an ideal gas is S = Nkg In V. Entropy is a state function rather than a path function, and in this problem, you will show an example of the entropy change for an ideal gas being the same when you go between the same two states by two different pathways. A. Express ASV = S2 (V2) - S₁(V1), the change in entropy upon changing the volume from V₁to V2, at fixed particle number N and energy, U. B. Express ASN = S₂(N₂) - S₁ (N₁), the change in entropy upon changing the particle number from N₁ to N2, at fixed volume V and energy U. C. Write an expression for the entropy change, AS, for a two-step process (V₁, N₁) → (V2, N₁) → (V2, N₂) in which the volume changes first at fixed particle number, then the particle number changes at fixed volume. Again, assume energy is constant.arrow_forwardPlease don't use Chatgpt will upvote and give handwritten solutionarrow_forward6. We used the constant volume heat capacity, Cv, when we talked about thermodynamic cycles. It acts as a proportionality constant between energy and temperature: dU = C₁dT. You can also define a heat capacity for constant pressure processes, Cp. You can think of enthalpy playing a similar role to energy, but for constant pressure processes δαρ C = (37) - Sup Ср ат P = ат Starting from the definition of enthalpy, H = U + PV, find the relationship between Cy and Cp for an ideal gas.arrow_forward
- Pure membranes of dipalmitoyl lecithin phospholipids are models of biological membranes. They melt = 41°C. Reversible melting experiments indicate that at Tm AHm=37.7 kJ mol-1. Calculate: A. The entropy of melting, ASm- B. The Gibbs free energy of melting, AGm- C. Does the membrane become more or less ordered upon melting? D. There are 32 rotatable CH2 CH2 bonds in each molecule that can rotate more freely if the membrane melts. What is the increase in multiplicity on melting a mole of bonds?arrow_forward5. Heat capacity often has a temperature dependence for real molecules, particularly if you go over a large temperature range. The heat capacity for liquid n-butane can be fit to the equation Cp(T) = a + bT where a = 100 J K₁₁ mol¹ and b = 0.1067 J K² mol¹ from its freezing point (T = 140 K) to its boiling point (T₁ = 270 K). A. Compute AH for heating butane from 170 K to 270 K. B. Compute AS for the same temperature range.arrow_forward4. How much energy must be transferred as heat to cause the quasi-static isothermal expansion of one mole of an ideal gas at 300 K from PA = 1 bar to PB = 0.5 bar? A. What is VA? B. What is VB? C. What is AU for the process? D. What is AH for the process? E. What is AS for the process?arrow_forward
- 1. The diagram shows the tube used in the Thomson experiment. a. State the KE of the electrons. b. Draw the path of the electron beam in the gravitational field of the earth. C. If the electric field directed upwards, deduce the direction of the magnetic field so it would be possible to balance the forces. electron gun 1KVarrow_forwardas a hiker in glacier national park, you need to keep the bears from getting at your food supply. You find a campground that is near an outcropping of ice. Part of the outcropping forms a feta=51.5* slopeup that leads to a verticle cliff. You decide that this is an idea place to hang your food supply out of bear reach. You put all of your food into a burlap sack, tie a rope to the sack, and then tie a bag full of rocks to the other end of the rope to act as an anchor. You currently have 18.5 kg of food left for the rest of your trip, so you put 18.5 kg of rocks in the anchor bag to balance it out. what happens when you lower the food bag over the edge and let go of the anchor bag? Determine the acceleration magnitude a of the two-bag system when you let go of the anchor bag?arrow_forward2. A thin Nichrome wire is used in an experiment to test Ohm's law using a power supply ranging from 0 to 12 V in steps of 2 V. Why isn't the graph of I vs V linear? 1. Nichrome wire does obey Ohm's law. Explain how that can that be true given the results abovearrow_forward
- 1. The average KE and temperature in Kelvin of the molecules of a gas are related by the equation KE = 3/2 KT where k is the Boltzmann constant 1.38 x 10 m² kg s². The diagram shows the energy levels for a Hydrogen atom. Energy/eV 0.00 -1.51 3.39 13.58 Use this information to show that Hydrogen at room temperature will not emit light. 2. When hydrogen burns in oxygen 241.8 kJ of energy are released per mole. Show that this reaction can produce light.arrow_forward3. By using the fact that around any closed loop the sum of the EMFS = the sum of the PDs. Write equations for the two loops shown in the cct below. 40 ΔΩ I₂ 4V (loop1 20 (loop2) 2v I+12 Use these equations to show that the current flowing through the 20 resistor is 0.75Aarrow_forward5. A potential divider circuit is made by stretching a 1 m long wire with a resistance of 0.1 per cm from A to B as shown. 8V A 100cm B sliding contact 5Ω A varying PD is achieved across the 5 Q resistor by moving the slider along the resistance wire. Calculate the distance from A when the PD across the 5 Q resistor is 6 V.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
 Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning


Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Kinetic Energy and Potential Energy; Author: Professor Dave explains;https://www.youtube.com/watch?v=g7u6pIfUVy4;License: Standard YouTube License, CC-BY