
EBK ORGANIC CHEMISTRY
12th Edition
ISBN: 9781119233664
Author: Snyder
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 20P
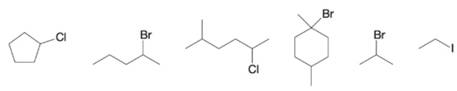
Which of the following compounds can be prepared by radical halogenations which little complication by formation of isomeric by-products?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Leucine is an essential amino acid with the systematic name 2-amino-3-methylpentanoic acid. It has pai
2.36 and pKa2 = 9.60.
H2N-C(R)H-COOH and R is -CH2-CH(CH3)2
A. Draw the condensed structure for leucine, and label all chirality centers with an asterisk.
B. How many possible stereoisomers of leucine are there?
C. Draw a Fischer projection of L-leucine and label the chirality center(s) as R or S.
D. What is the p/ of leucine?
E. Draw the structure of the predominant form of leucine at 10.00.
F.
Draw the structure of the predominant form of leucine at pH = 1.50.
G. Leucine is described as an essential amino acid. What does this mean?
H. Show the alkyl halide you would use to prepare leucine by the amidomalonate method.
=
a) Write out 6 completely different reactions of acetophenone (reagent, product).
b) Write out 3 preparations of 1-methylcyclohexanol, using a different starting material
for each one. You may use preps where you just change the functional group, and/or
preps where you construct the carbon chain.
c) Write out 3 preparations of 2-ethoxybenzoic acid, a different starting material for
each one. You may use preps where you just change the functional group, and/or
preps where you construct the carbon chain.
12.
CH3
OH
OH
H&C
CH3
H₂C
N
OH H₂C
CH3
H&C
CH3 H₂C'
CH3
H.C
CH3OH
H.C
CH2CH3OH
CH3CEN
Which one of these 17 compounds is represented by this IR and this 'H NMR
spectrum?
IR Spectrum
3000
4000
3000
NMR Spectrum
2000
£500
RAVENUMBER
2000
1500
9
8
6
5
10
HP-00-290
ppm
m
1000
500
1000
4
°
Chapter 10 Solutions
EBK ORGANIC CHEMISTRY
Ch. 10 - Prob. 1PPCh. 10 - Prob. 2PPCh. 10 - Practice Problem 10.3 How would the molecular ion...Ch. 10 - Prob. 4PPCh. 10 - Prob. 5PPCh. 10 - Prob. 6PPCh. 10 - Practice Problem 10.7 Chlorination reactions of...Ch. 10 - Prob. 8PPCh. 10 - Prob. 9PPCh. 10 - Prob. 10PP
Ch. 10 - Prob. 11PPCh. 10 - Practice Problem 10.12 Benzylic radicals, due to...Ch. 10 - Prob. 13PPCh. 10 - Practice Problem 10.14 Show how the following...Ch. 10 - Prob. 15PPCh. 10 - Prob. 16PPCh. 10 - Prob. 17PPCh. 10 - Prob. 18PCh. 10 - Explain the relative distribution of produces...Ch. 10 - 10.20 Which of the following compounds can be...Ch. 10 - Prob. 21PCh. 10 - Prob. 22PCh. 10 - Prob. 23PCh. 10 - Prob. 24PCh. 10 - 10.25 List in order of decreasing stability all of...Ch. 10 - Prob. 26PCh. 10 - Prob. 27PCh. 10 - Prob. 28PCh. 10 - Starting with the compound or compounds indicated...Ch. 10 - Prob. 30PCh. 10 - 10.31 Synthesize each of the following compounds...Ch. 10 - Synthesize each of die following compounds by...Ch. 10 - Prob. 33PCh. 10 - Prob. 34PCh. 10 - Prob. 36PCh. 10 - The halogen atom of an alkyl halide can be...Ch. 10 - Prob. 38PCh. 10 - Prob. 39PCh. 10 - Write a mechanism for the following reaction.Ch. 10 - 10.41 Hydrogen peroxide and ferrous sulfate react...Ch. 10 - Prob. 42PCh. 10 - If one were to try to draw the simplest Lewis...Ch. 10 - Prob. 1LGPCh. 10 - 2. (a) Propose a synthesis of 2-methoxypropene...
Additional Science Textbook Solutions
Find more solutions based on key concepts
47. A 250.0-mL buffer solution is 0.250 M in acetic acid and 0.250 M in sodium acetate.
a. What is the initial ...
Chemistry: A Molecular Approach (4th Edition)
The bioremediation process shown in the photograph is used to remove benzene and other hydrocarbons from soil c...
Microbiology: An Introduction
Examine the following diagrams of cells from an organism with diploid number 2n = 6, and identify what stage of...
Genetic Analysis: An Integrated Approach (3rd Edition)
SCIENTIFIC INQUIRY You are handed a mystery pea plant with tall stems and axial flowers and asked to determine ...
Campbell Biology (11th Edition)
8. A classic way to isolate thymidylate synthase—negative mutants of bacteria is to treat a growing culture wit...
Biochemistry: Concepts and Connections (2nd Edition)
The similarity and difference in ionic and covalent bond should be determined. Concept Introduction: The intera...
Living By Chemistry: First Edition Textbook
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the structure of (E,6R) 6-methoxy-4-hepten-2-one. Give the IUPAC name of this compound, including stereochemistry. Draw the most stable chair conformation of (cis) 1,3-isobutylcyclohexane. H HC=CCH₂ CH2CH3 EN(CH3)2 -CN(CH3)2arrow_forward10. Write out the mechanism (intermediate/transition state) for this reaction; indicate stereochemistry in product. H3C CH₂OH CH3 SN1 Harrow_forwardWrite "most" under the member of each trio which is most stable. Write "least under the member of each trio which is least stable. b) Draw a Fischer projection of a pair of enantiomers with three chiral carbons. Which of these two would you expect to be more soluble in water? Why? 1-butanol 1-heptanol Which of these two would you expect to have the higher boiling point? Why? hexyl methyl ether 1-heptanolarrow_forward
- Write "most" under the most acidic compound. Write "least" under the least acidic compound. OH NO₂ OCH3 Br 9. Compound X, C50H84F2, reacts with excess H2/Pd to give a C50H88F2 compound. How many rings are in X? How many double bonds are in X? Show your work.arrow_forward4. State whether these two are: a) the same molecule b) c) d) different compounds that are not isomers constitutional isomers diastereomers e) enantiomers CH3 CH₁₂ H OH HO H H OH HO H CH, CH₂ 5. a) How many stereocenters does this compound have? b) How many stereoisomers are possible for this compound? CH₂ OH CHCHarrow_forwardCalculating the pH at equivalence of a titration A chemist titrates 210.0 mL of a 0.1003 M hydrobromic acid (HBr) solution with 0.7550M KOH solution at 25 °C. Calculate the pH at equivalence. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = ] ☑ o0o 18 Ararrow_forward
- Do you do chemistry assignmentsarrow_forwardUsing the conditions of spontaneity to deduce the signs of AH and AS Use the observations about each chemical reaction in the table below to decide the sign (positive or negative) of the reaction enthalpy AH and reaction entropy AS. Note: if you have not been given enough information to decide a sign, select the "unknown" option. reaction observations conclusions A This reaction is always spontaneous, but proceeds slower at temperatures above 120. °C. ΔΗ is (pick one) AS is (pick one) ΔΗ is (pick one) B This reaction is spontaneous except above 117. °C. AS is (pick one) ΔΗ is (pick one) This reaction is slower below 20. °C than C above. AS is |(pick one) ? 18 Ar 1arrow_forwardCalculating the pH at equivalence of a titration Try Again Your answer is incorrect. 0/5 a A chemist titrates 70.0 mL of a 0.7089 M hydrocyanic acid (HCN) solution with 0.4574M KOH solution at 25 °C. Calculate the pH at equivalence. The pK of hydrocyanic acid is 9.21. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = 11.43] G 00. 18 Ar B•arrow_forward
- Biological Macromolecules Naming and drawing the products of aldose oxidation and reduction aw a Fischer projection of the molecule that would produce L-ribonic acid if it were subjected to mildly oxidizing reaction conditions. Click and drag to start drawing a structure. X AP ‡ 1/5 Naor Explanation Check McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibilarrow_forward● Biological Macromolecules Identifying the parts of a disaccharide Take a look at this molecule, and then answer the questions in the table below it. CH2OH O H H H OH OH OH H H CH2OH H O OH H OH H H H H OH Is this a reducing sugar? Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. Explanation Check O yes X O no ○ yes O no Uarrow_forwardThe aim of the lab is to measure the sodium content from tomato sauce using the Mohr titration method. There are two groups being: Regular Tomato sauce & Salt Reduced tomato sauce QUESTION: State how you would prepare both Regular & Salt reduced tomato sauce samples for chemical analysis using the Mohr titration methodarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License