
Concept explainers
(a)
Interpretation:
A Lewis structure for
Concept introduction:
Lewis structure is generally considered as a simplified structure of any molecule or atom. Lewis structure for any atom or molecule depicts the valence electrons as dots around the element’s symbol present in the molecule along with the bonds that connect them. Every element tries to complete an octet except the hydrogen atom.
Every element in the Lewis structure tries to attain eight electrons in its valence shell by transfer or share of electrons. This rule is known as the octet rule.
To draw the Lewis structure of the molecule there are following steps:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Step 5: Convert the lone pair into bond pair.
(a)
Answer to Problem 10.76P
The Lewis structure for
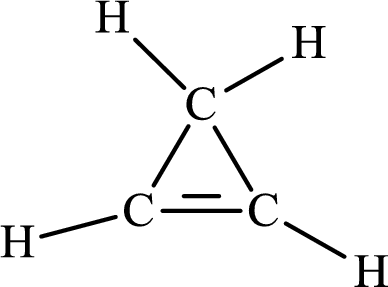
The carbon-carbon single bond has the bond order 1 and carbon-carbon double bond has the bond order 2.
Explanation of Solution
The total number of valence electrons of
Substitute 3 for the total number of
In
The Lewis structure of
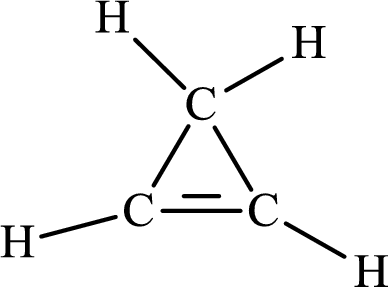
The single bond has the bond order 1 and double bond has the bond order 2. Therefore, the carbon-carbon single bond has the bond order 1 and carbon-carbon double bond has the bond order 2.
The single bond has the bond order 1, the double bond has the bond order 2 and triple bond has the bond order 3.
(b)
Interpretation:
A Lewis structure for
Concept introduction:
Lewis structure is generally considered as a simplified structure of any molecule or atom. Lewis structure for any atom or molecule depicts the valence electrons as dots around the element’s symbol present in the molecule along with the bonds that connect them. Every element tries to complete an octet except the hydrogen atom.
Every element in the Lewis structure tries to attain eight electrons in its valence shell by transfer or share of electrons. This rule is known as octet rule.
To draw the Lewis structure of the molecule there are following steps:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Step 5: Convert the lone pair into bond pair.
(b)
Answer to Problem 10.76P
The Lewis structure of
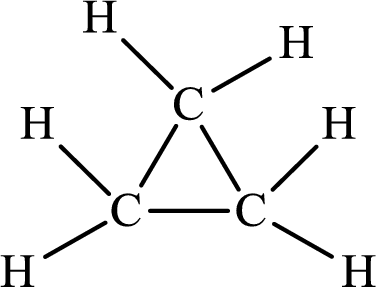
There is only single bond in
Explanation of Solution
The total number of valence electrons of
Substitute 3 for the total number of
In
The Lewis structure of
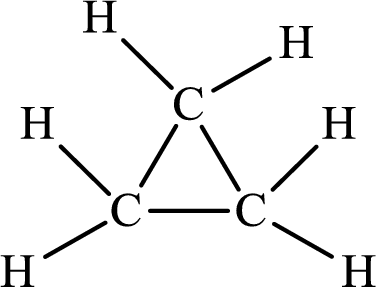
The single bond has the bond order 1 and double bond has the bond order 2. Therefore, there is only single bond in
The single bond has the bond order 1, double bond has the bond order 2 and triple bond has the bond order 3.
(c)
Interpretation:
A Lewis structure for
Concept introduction:
Lewis structure is generally considered as a simplified structure of any molecule or atom. Lewis structure for any atom or molecule depicts the valence electrons as dots around the element’s symbol present in the molecule along with the bonds that connect them. Every element tries to complete an octet except the hydrogen atom.
Every element in the Lewis structure tries to attain eight electrons in its valence shell by transfer or share of electrons. This rule is known as the octet rule.
To draw the Lewis structure of the molecule there are following steps:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Step 5: Convert the lone pair into bond pair.
(c)
Answer to Problem 10.76P
The Lewis structure of
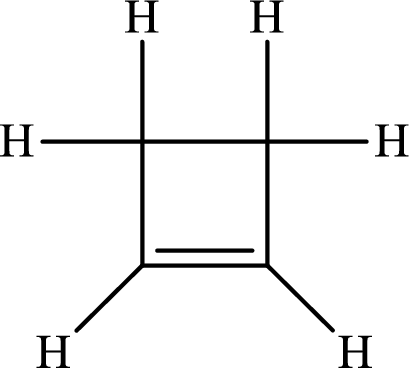
The carbon-carbon single bond has the bond order 1 and carbon-carbon double bond has the bond order 2.
Explanation of Solution
The total number of valence electrons of
Substitute 4 for the total number of
In
The Lewis structure of
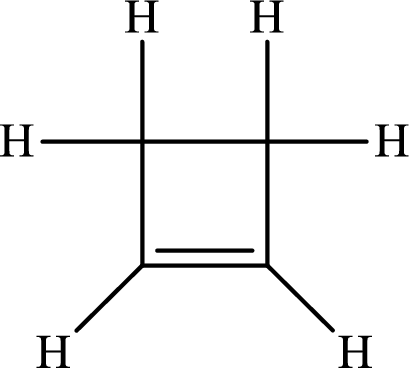
The single bond has the bond order 1 and double bond has the bond order 2. Therefore, the carbon-carbon single bond has the bond order 1 and carbon-carbon double bond has the bond order 2.
The single bond has the bond order 1, the double bond has the bond order 2 and triple bond has the bond order 3.
(d)
Interpretation:
A Lewis structure for
Concept introduction:
Lewis structure is generally considered as a simplified structure of any molecule or atom. Lewis structure for any atom or molecule depicts the valence electrons as dots around the element’s symbol present in the molecule along with the bonds that connect them. Every element tries to complete an octet except the hydrogen atom.
Every element in the Lewis structure tries to attain eight electrons in its valence shell by transfer or share of electrons. This rule is known as the octet rule.
To draw the Lewis structure of the molecule there are following steps:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Step 5: Convert the lone pair into bond pair.
(d)
Answer to Problem 10.76P
The Lewis structure of
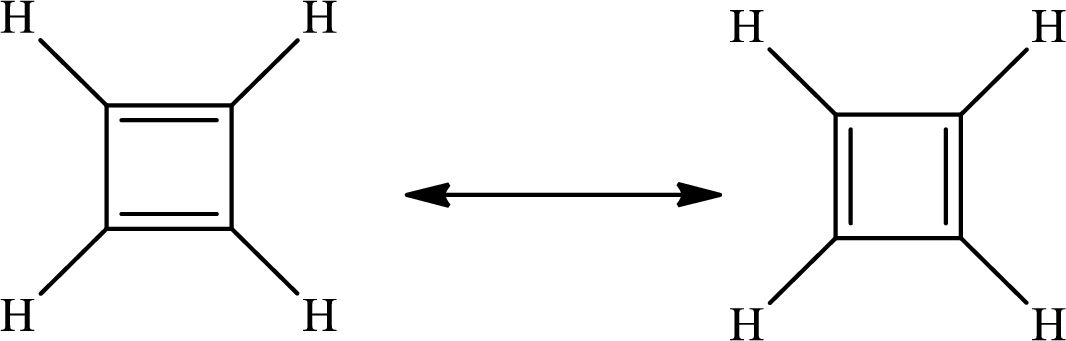
The average bond order in
Explanation of Solution
The total number of valence electrons of
Substitute 4 for the total number of
In
There is alternate single and double present in the ring so the molecule will show resonance.
The Lewis structure of
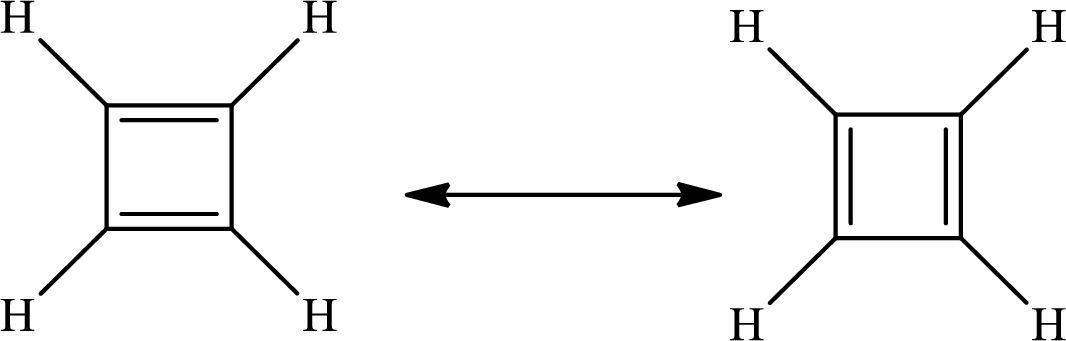
There are 4 single bonds and 2 double bonds in
The formula to calculate the average bond order in
Substitute 6 for the number of shared electron pair and 4 for the number of bonded atoms in equation (5).
The single bond has the bond order 1, the double bond has the bond order 2 and triple bond has the bond order 3.
(e)
Interpretation:
A Lewis structure for
Concept introduction:
Lewis structure is generally considered as a simplified structure of any molecule or atom. Lewis structure for any atom or molecule depicts the valence electrons as dots around the element’s symbol present in the molecule along with the bonds that connect them. Every element tries to complete an octet except the hydrogen atom.
Every element in the Lewis structure tries to attain eight electrons in its valence shell by transfer or share of electrons. This rule is known as the octet rule.
To draw the Lewis structure of the molecule there are following steps:
Step 1: Find the central atom and place the other atoms around it. The atom in a compound which has the lowest group number or lowest electronegativity considered as the central atom.
Step 2: Calculate the total number of valence electrons.
Step 3: Connect the other atoms around the central atoms to the central atom with a single bond and lower the value of valence electrons by 2 of every single bond.
Step 4: Allocate the remaining electrons in pairs so that each atom can get 8 electrons.
Step 5: Convert the lone pair into bond pair.
(e)
Answer to Problem 10.76P
The Lewis structure of
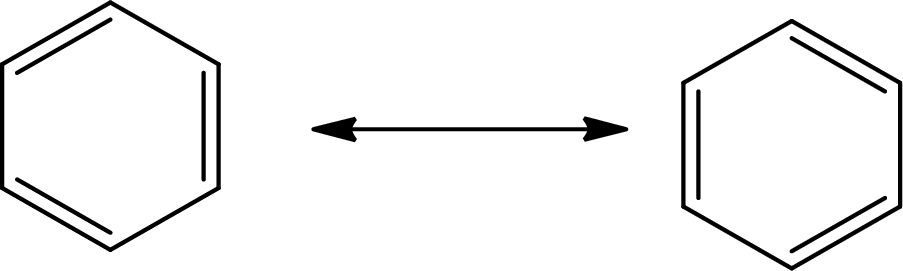
The average bond order in
Explanation of Solution
The total number of valence electrons of
Substitute 6 for the total number of
In
There is alternate single and double present in the ring so the molecule will show resonance.
The Lewis structure of
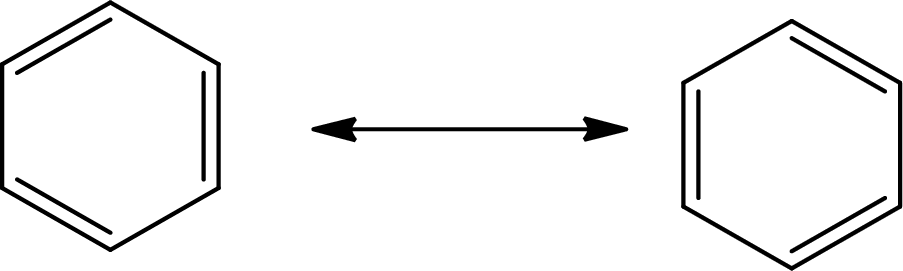
There are 6 single bonds and 3 double bonds in
The formula to calculate the average bond order in
Substitute 9 for the number of shared electron pair and 6 for the number of bonded atoms in equation (7).
The single bond has the bond order 1, the double bond has the bond order 2 and triple bond has the bond order 3.
Want to see more full solutions like this?
Chapter 10 Solutions
CHEM 212:CHEMISTSRY V 2
- 2. Provide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases. a. CH3arrow_forwardIdentify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forward
- Instructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forwardе. Д CH3 D*, D20arrow_forwardC. NaOMe, Br Brarrow_forward
- Please predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forwardin the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





