
Concept explainers
(a)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
The mechanism for epoxides under neutral or basic conditions consists of an
Answer to Problem 10.53P
The product of the given reaction is:
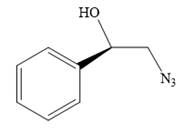
Explanation of Solution
The given reaction is:
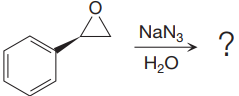
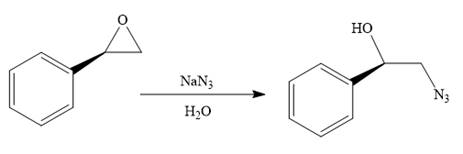
When the given epoxide is treated with sodium azide (basic conditions), the azide ion in sodium azide acts as a nucleophile and attacks the less substituted carbon atom (highlighted in red) of the epoxide. Due to this, the highly strained epoxide ring opens and the azide gets attached to the less substituted carbon atom. In the presence of a solvent such as water, the negatively charged oxygen atom is protonated resulting in the final product. The product of the reaction when the given epoxide is treated with sodium azide is as below:
Epoxides can undergo nucleophilic substitution reactions under neutral or basic conditions in which the nucleophile attacks the least substituted carbon atom in the epoxide.
(b)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
The mechanism for the epoxides under neutral or basic conditions consists of an
Answer to Problem 10.53P
The product of the given reaction is:
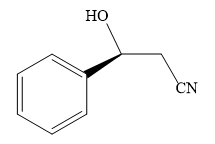
Explanation of Solution
The given reaction is:
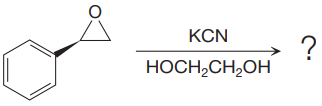
The given reaction conditions are basic as the potassium cyanide is a weak base and nucleophile.
When the given epoxide is treated with potassium cyanide (neutral conditions), the cyanide ion acts as a nucleophile and attacks the least substituted carbon atom of the epoxide. Due to this, the highly strained epoxide ring opens and the cyanide ion gets attached to the carbon. In the presence of a solvent such as ethanediol, the negatively charged oxygen atom is protonated resulting in the final product. The product of the reaction when the given epoxide is treated with sodium azide is as below:
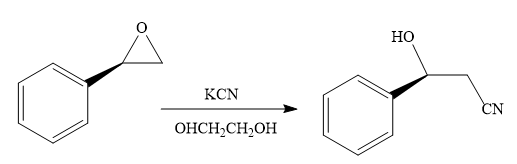
Epoxides can undergo nucleophilic substitution reactions under neutral or basic conditions in which the nucleophile attacks the least substituted carbon atom in the epoxide.
(c)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
Under acidic conditions, the first step is the protonation of
Answer to Problem 10.53P
The product of the given reaction is:
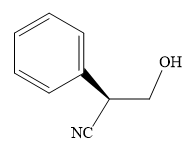
Explanation of Solution
The given reaction is:
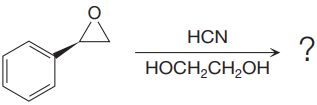
The given reaction conditions are acidic as hydrogen cyanide is a weak acid.
When the given epoxide is treated with hydrogen cyanide (acidic conditions), the first step is the protonation of the epoxide oxygen atom. Due to this, a partial positive charge is generated on both carbon atoms in the epoxide. Out of the two carbon atoms, the one that is most substituted would be able to stabilize the partially developed charge, and thus, in the second step, the nucleophile, cyanide ion attacks the most substituted carbon atom from the side opposite to the
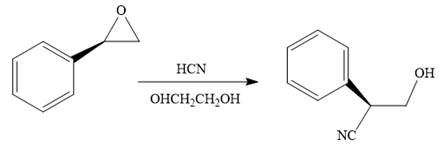
Epoxides can undergo nucleophilic substitution reactions under acidic conditions in which the nucleophile attacks the most substituted carbon atom in the epoxide.
(d)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
The mechanism for the epoxides under neutral or basic conditions consists of an
Answer to Problem 10.53P
The product of the given reaction is:
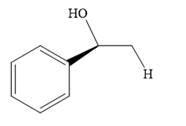
Explanation of Solution
The given reaction is:
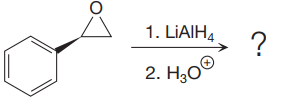
The given reaction conditions are basic as lithium aluminum hydride is a strong base.
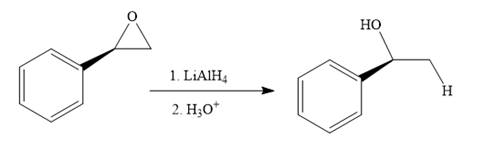
Epoxides can undergo nucleophilic substitution reactions under neutral or basic conditions in which the nucleophile attacks the least substituted carbon atom in the epoxide.
(e)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
Under acidic conditions, the first step is the protonation the
Answer to Problem 10.53P
The product of the given reaction is:
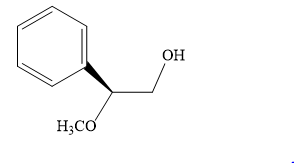
Explanation of Solution
The given reaction is:
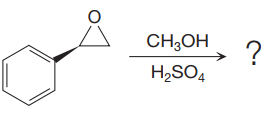
The given reaction conditions are methanol in sulfuric acid. Sulfuric acid is a strong acid. When it is treated with methanol, it will produce protonated methanol which is a strongest known acid. When the given epoxide is treated with protonated methanol, (highly acidic conditions), the first step is the protonation of the oxygen atom in the epoxide. Due to this, a partial positive charge is generated on both the carbon atoms in the epoxide. Out of the two carbon atoms, the one that is most substituted would be able to stabilize the partially developed positive charge. Thus, in the second step, the nucleophile, methoxide ion attacks the most substituted carbon atom from the side opposite to the protonated
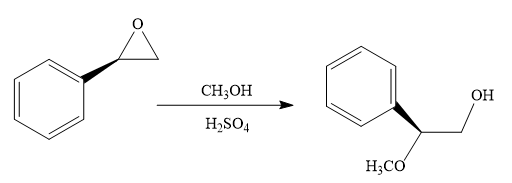
Epoxides can undergo nucleophilic substitution reactions under acidic conditions in which the nucleophile attacks on the most substituted carbon atom in the epoxide.
(f)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
The mechanism for the epoxides under neutral or basic conditions consists of an
Answer to Problem 10.53P
The product of the given reaction is:
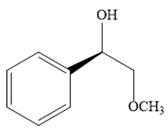
Explanation of Solution
The given reaction is:
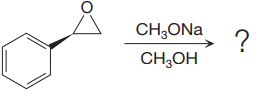
When the given epoxide is treated with sodium methoxide in methanol (basic conditions), the methoxide ion acts as a nucleophile and attacks the least substituted carbon atom of the epoxide. Due to this, the highly strained epoxide ring opens and the methoxide ion gets attached to the least substituted carbon atom. The product of the reaction when the given epoxide is treated with sodium methoxide in methanol is as below:
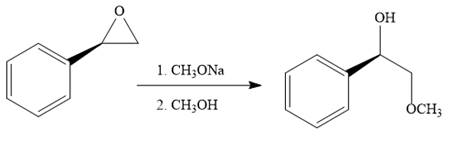
Epoxides can undergo nucleophilic substitution reactions under neutral or basic conditions in which the nucleophile attacks the least substituted carbon atom in the epoxide.
(g)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
The mechanism for the epoxides under neutral or basic conditions consists of an
Answer to Problem 10.53P
The product of the given reaction is:
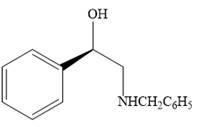
Explanation of Solution
The given reaction is:
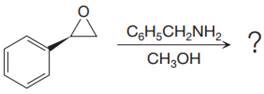
When the given epoxide is treated with benzyl
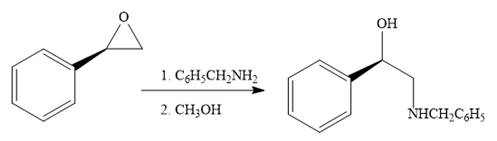
Epoxides can undergo nucleophilic substitution reactions under neutral or basic conditions in which the nucleophile attacks on the least substituted carbon atom in the epoxide.
(h)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
The mechanism for the epoxides under neutral or basic conditions consists of an
Answer to Problem 10.53P
The product of the given reaction is:
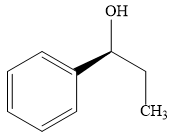
Explanation of Solution
The given reaction is:
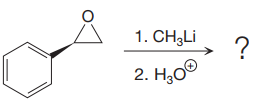
Methyl lithium is an inorganic methylating agent. When the given epoxide is treated with methyl lithium (basic conditions), the methyl anion acts as a nucleophile and attacks the least substituted carbon atom of the epoxide. Due to this, the highly strained epoxide ring opens. In the second step, acidic workup is important so as to protonate the negatively charged oxygen atom.
The product of the reaction when the given epoxide is treated with methyl lithium in the acidic workup is as below:
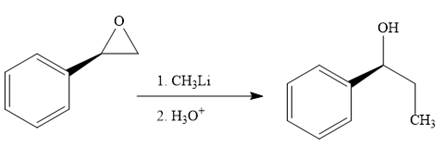
Epoxides can undergo nucleophilic substitution reactions under neutral or basic conditions in which the nucleophile attacks the least substituted carbon atom in the epoxide.
Want to see more full solutions like this?
Chapter 10 Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely: (a) 0.000259 M HClO4arrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardDetermine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. NaN₃arrow_forward
- A. Draw the structure of each of the following alcohols. Then draw and name the product you would expect to produce by the oxidation of each. a. 4-Methyl-2-heptanol b. 3,4-Dimethyl-1-pentanol c. 4-Ethyl-2-heptanol d. 5,7-Dichloro-3-heptanolarrow_forwardWhat is the pH of a 1.0 L buffer made with 0.300 mol of HF (Ka = 6.8 × 10⁻⁴) and 0.200 mol of NaF to which 0.160 mol of NaOH were added?arrow_forwardCan I please get help with this.arrow_forward
- Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, simply write only NR. Be sure to include the proper phases for all species within the reaction. N₂H₅ClO₄arrow_forwardPlease help me with identifying these.arrow_forwardCan I please get help with this?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

