
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 10, Problem 10.51AP
Interpretation Introduction
Interpretation:
The definition for 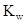 and the numerical value at
and the numerical value at 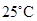 should be determined.
should be determined.
Concept introduction:
Ionic-product constant for water: It is the hydronium ion concentration times the 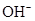 concentration present in the solution.
concentration present in the solution.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
what are the different classes and some examples of neuroprotectants that can be used to treat, prevent, or combat neurotoxicity/a neurotoxicant...for example, antioxidants, nutraceuticals, etc.,..?
Imagine that aldolase can react with the seven carbon molecule Sedoheptulose-1,7-bisphosphate (below). Use the mechanism to predict the two products generated.
Please draw out the stereochemistry in a fischer projection.
Sodium borohydride (NaBH4) is a potent inhibitor of aldolase. It is known to ONLY inhibit theenzyme when it is complexed with substrate. Treatment of the enzyme alone has no effect.What is the mechanism for this inhibition?
Please draw out the mechanism and show how it inhibits this.
Chapter 10 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 10.1 - Which of the following are BrnstedLowry acids?...Ch. 10.1 - Prob. 10.2PCh. 10.1 - Prob. 10.3PCh. 10.1 - Prob. 10.4KCPCh. 10.2 - The concentration of HCl when released to the...Ch. 10.2 - Prob. 10.2CIAPCh. 10.2 - Prob. 10.3CIAPCh. 10.2 - Prob. 10.5PCh. 10.2 - Prob. 10.6PCh. 10.2 - Prob. 10.7P
Ch. 10.2 - Prob. 10.8PCh. 10.2 - Prob. 10.9KCPCh. 10.3 - Prob. 10.10PCh. 10.4 - Prob. 10.11PCh. 10.5 - Prob. 10.12PCh. 10.5 - Prob. 10.13PCh. 10.5 - Prob. 10.14PCh. 10.6 - Identify the following solutions as acidic or...Ch. 10.6 - Calculate the pH of the following solutions and...Ch. 10.6 - What is the pH of a 0.0025 M solution of HCl?Ch. 10.6 - Prob. 10.4CIAPCh. 10.6 - Prob. 10.5CIAPCh. 10.7 - How many equivalents are in the following? (a) 5.0...Ch. 10.7 - Prob. 10.19PCh. 10.8 - Maalox, an over-the-counter antacid, contains...Ch. 10.8 - Prob. 10.21PCh. 10.8 - Prob. 10.22PCh. 10.8 - Show how ethylamine (C2H5NH2) reacts with...Ch. 10.9 - Predict whether the following salts produce an...Ch. 10.10 - What is the pH of 1.00 L of the 0.100 M...Ch. 10.10 - Prob. 10.26PCh. 10.10 - Prob. 10.27PCh. 10.10 - A buffer solution is prepared using CN-(from NaCN...Ch. 10.11 - A titration is carried out to determine the...Ch. 10.11 - Prob. 10.30PCh. 10.11 - Prob. 10.31PCh. 10.11 - Prob. 10.32PCh. 10.11 - Prob. 10.6CIAPCh. 10.11 - Prob. 10.7CIAPCh. 10 - Prob. 10.33UKCCh. 10 - Prob. 10.34UKCCh. 10 - The following pictures represent aqueous acid...Ch. 10 - Prob. 10.36UKCCh. 10 - Prob. 10.37UKCCh. 10 - Prob. 10.38APCh. 10 - What happens when a weak acid such as CH3CO2H is...Ch. 10 - What happens when a strong base such as KOH solved...Ch. 10 - Prob. 10.41APCh. 10 - Prob. 10.42APCh. 10 - Prob. 10.43APCh. 10 - Prob. 10.44APCh. 10 - Prob. 10.45APCh. 10 - Prob. 10.46APCh. 10 - Label the BrnstedLowry acids and bases in the...Ch. 10 - Write the formulas of the conjugate acids of the...Ch. 10 - Write the formulas of the conjugate bases of the...Ch. 10 - Prob. 10.50APCh. 10 - Prob. 10.51APCh. 10 - Prob. 10.52APCh. 10 - Prob. 10.53APCh. 10 - Prob. 10.54APCh. 10 - Write the expressions for the acid dissociation...Ch. 10 - Based on the Ka values in Table 10.3, rank the...Ch. 10 - Prob. 10.57APCh. 10 - A 0.10 M solution of the deadly poison hydrogen...Ch. 10 - Prob. 10.59APCh. 10 - Prob. 10.60APCh. 10 - What is the approximate pH of a 0.02 M solution of...Ch. 10 - Calculate the pOH of each solution in Problems...Ch. 10 - Prob. 10.63APCh. 10 - What are the OH concentration and pOH for each...Ch. 10 - What are the H3O+ and OH concentrations of...Ch. 10 - Prob. 10.66APCh. 10 - Prob. 10.67APCh. 10 - Write balanced equations for proton-transfer...Ch. 10 - Sodium bicarbonate (NaHCO3), also known as baking...Ch. 10 - Refer to Section 10.8 to write balanced equations...Ch. 10 - Prob. 10.71APCh. 10 - For each of the following salts, indicate if the...Ch. 10 - Which salt solutions in problem 10.72 could be...Ch. 10 - Prob. 10.74APCh. 10 - Prob. 10.75APCh. 10 - Prob. 10.76APCh. 10 - Which of the following buffer systems would you...Ch. 10 - What is the pH of a buffer system that contains...Ch. 10 - Consider 1.00 L of the buffer system described in...Ch. 10 - Prob. 10.80APCh. 10 - Prob. 10.81APCh. 10 - Prob. 10.82APCh. 10 - How does normality compare to molarity for...Ch. 10 - Prob. 10.84APCh. 10 - Prob. 10.85APCh. 10 - Prob. 10.86APCh. 10 - Prob. 10.87APCh. 10 - Prob. 10.88APCh. 10 - Prob. 10.89APCh. 10 - Prob. 10.90APCh. 10 - Prob. 10.91APCh. 10 - Titration of a 12.0 mL solution of HCl requires...Ch. 10 - Prob. 10.93APCh. 10 - Titration of a 10.0 mL solution of NH3 requires...Ch. 10 - If 35.0 mL of a 0.100 N acid solution is needed to...Ch. 10 - For the titrations discussed in Problems 10.92 and...Ch. 10 - Prob. 10.97APCh. 10 - Prob. 10.98CPCh. 10 - Prob. 10.99CPCh. 10 - Prob. 10.100CPCh. 10 - Prob. 10.101CPCh. 10 - Prob. 10.102CPCh. 10 - Prob. 10.103CPCh. 10 - Prob. 10.104CPCh. 10 - Prob. 10.105CPCh. 10 - Prob. 10.106CPCh. 10 - Prob. 10.107CPCh. 10 - Prob. 10.108CPCh. 10 - Obtain a package of Alka-Seltzer, an antacid, from...Ch. 10 - Prob. 10.110GPCh. 10 - Prob. 10.111GP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Show the fate of the proton on the 4-Oxygen molecule of F-1,6-BP. Please include a drawing showing the electron flow that occurs.arrow_forward1. Which one is the major organic product obtained from the following aldol condensation? O NaOH, H₂O heat A B C D Earrow_forwardAn organic chemist ordered the wrong item. She wanted to obtain 1-hydroxy-2-butanone, butinstead ordered 2-hydroxybutyraldehyde. As a good biochemist, show how the organic chemistcould use biological catalysis to make her desired compound. Please show the mechanism by drawing.arrow_forward
- Show the fate of the hydrogen on carbon-2 of glucose. Please draw out the structure using curve arrows to show electron flow.arrow_forward3. Which one of the compounds below is the major product formed by the reaction sequence shown here? CH3 + CH3NO2 NaOH H2, Ni ? nitromethane acetophenone OH OH HO HN- u x x x x Ph A HO -NH2 HO H Ph Ph Ph N- H B Ph NH2 D Earrow_forward4. Only ONE of the five compounds below can be prepared by an aldol condensation in which a single carbonyl compound is treated with base. Which one is it? To solve this problem, reverse the aldol condensation that formed each of these molecules to find out what two molecules came together to make the products. The one in which the two molecules are identical is the answer. Ph Ph ཚིག གནས ག ནཱ ཀ ན ཀནཱ A Ph H B Ph Ph H D Ph. Ph Ph E Harrow_forward
- 5. Which one is the major organic product obtained from the following reaction sequence? First, equimolar amounts of cyclopentanone and LDA are mixed at -78°C. Then propionaldehyde (propanal) is added. Addition of aqueous acid completes the process. LDA, -78°C. 1. 2. H₂O* H A B H 0 D H H Earrow_forward2. Which one is the major organic product obtained from the following reaction? NaOH, H₂O heat A B C D Earrow_forwardCH3CH2CHO + propanal PhCH2CHO 2-phenylacetaldehyde mixture of four products NaOH 10. In the crossed aldol reaction of propanal and 2-phenylacetaldehyde shown above, a mixture of four products will be formed. Which ONE of the compounds below will NOT be formed in this crossed aldol reaction? OH Ph A H OH OH Ph H B OH OH H H H Ph Ph C Ph D Earrow_forward
- An organic chemist ordered the wrong item. She wanted to obtain 1-hydroxy-2-butanone, butinstead ordered 2-hydroxybutyraldehyde. As a good biochemist, show how the organic chemistcould use biological catalysis to make her desired compound.arrow_forwardPredict the products of aldolase catalyzing the reaction with acetone and (S)-3-hydroxybutyraldehyde.arrow_forwardA cancer patient undergoing chemotherapy is taking a protein kinase inhibitor drug. The patientis an avid marathon runner and does not want to miss his upcoming race. Is it a good idea forthis patient to attempt a marathon while on this medication? Explain why or why not.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning

Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning





GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license