
Concept explainers
(a)
Interpretation:
The given Lewis structure needs to be completed.
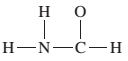
Concept Introduction:
The Lewis dot structure is the representation of a molecule or compound in which atoms are arranged or bonded in such a way that all the atoms have complete octets.
The bond formation between the atoms takes place due to the sharing of valence electrons between them while the remaining outer electrons are denoted as lone pair of electrons.
(b)
Interpretation:
The given Lewis structure needs to be completed.
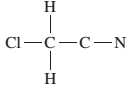
Concept Introduction:
The Lewis dot structure is the representation of a molecule or compound in which atoms are arranged or bonded in such a way that all the atoms have complete octets.
The bond formation between the atoms takes place due to the sharing of valence electrons between them while the remaining outer electrons are denoted as lone pair of electrons.
(c)
Interpretation:
The given Lewis structure needs to be completed.
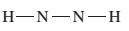
Concept Introduction:
The Lewis dot structure is the representation of a molecule or compound in which atoms are arranged or bonded in such a way that all the atoms have complete octets.
The bond formation between the atoms takes place due to the sharing of valence electrons between them while the remaining outer electrons are denoted as lone pair of electrons.
(d)
Interpretation:
The given Lewis structure needs to be completed.
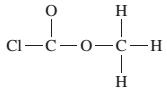
Concept Introduction:
The Lewis dot structure is the representation of a molecule or compound in which atoms are arranged or bonded in such a way that all the atoms have complete octets.
The bond formation between the atoms takes place due to the sharing of valence electrons between them while the remaining outer electrons are denoted as lone pair of electrons.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
EP BASIC CHEMISTRY-STANDALONE ACCESS
- Draw the mechanism (including all curved arrows for electron movement) showing how the maleicanhydride is attacked by the anthracene and formation of the final Diels Alder product.arrow_forwardProvide the missing information. *see imagearrow_forwardProvide the missing information. *see imagearrow_forward
- Provide the missing information. *see imagearrow_forwardI have a bottle of butanal that has been improperly used by lab workers. They allowed a traceamount NaOH (aq) to contaminate the bottle. What is now in my bottle of “butanal? What is the molecular name and functional group name? Draw the structure.arrow_forwardProvide the missing information. *see imagearrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning



