
The hydrogenation of ethylbenzene to ethylcyclohexane over a nickel-mordenite catalyst is zero order in both reactants up to an ethylbenzene conversion of 75% [Ind. Eng. Chem. Res., 28 (3), 260 (1989)]. At 553 K, k = 5.8 mol ethylbenzene/(dm3 of catalyst·h). When a 100-ppm thiophene concentration entered the system, the ethylbenzene conversion began to drop.
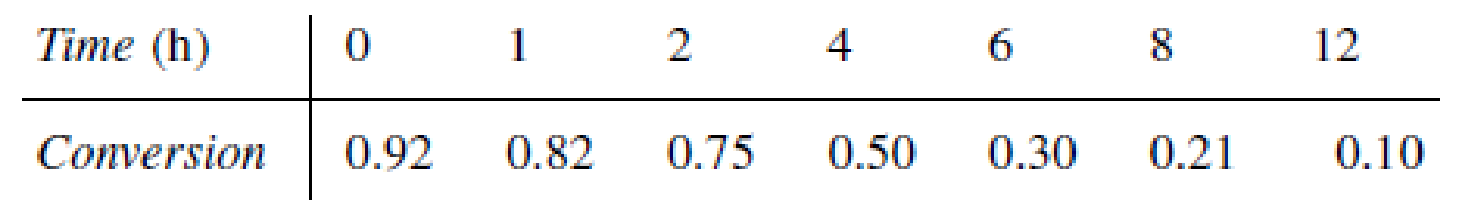
The reaction was carried out at 3 MPa and a molar ratio of H2/ETB = 10. Discuss the catalyst decay. Be quantitative where possible.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Additional Engineering Textbook Solutions
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
SURVEY OF OPERATING SYSTEMS
Java: An Introduction to Problem Solving and Programming (8th Edition)
Starting Out with Programming Logic and Design (5th Edition) (What's New in Computer Science)
Elementary Surveying: An Introduction To Geomatics (15th Edition)
- Use the binary diagram, 45 line above and material balance to solve the One thousand kg/h of a (50-50 wt%) acetone-in-water solution is to be extracted at 25C in a continuous, countercurrent system with pure 1,1,2-trichloroethane to obtain a raffinate containing 10 wt% acetone. Using the following equilibrium data, determine with an equilateral-triangle diagram: a the minimum flow rate of solvent; b. the number of stages required for a solvent rate equal to 1.5 times minimum, and composition of each streamleaving each stage. Repeat the calculation of (a) and (b) if the solvent used has purity 93wt% (4wr% acetone, 3wt% water impurities) acetone 0.6 water 0.13 1,1,2-trichloroethane 0.27 Raffinate. Weight Fraction Acetone Extract. Weight Fraction Acetone 0.5 0.04 0.46 0.44 0.56 0.4 0.03 0.57 0.29 0.40 0.3 0.02 0.681 0.12 0.18 0.2 0.015 0.785 0.0 0.0 0.1 0.01 0.89 0.55 0.35 0.1 0.5 0.43 0.07 0.4 0.57 0.03 0.3 0.68 0.02 0.2 0.79 0.01 0.1 0.895 0.005arrow_forwardMaterial Sciencearrow_forwardMaterial Sciencearrow_forward
- Material Sciencearrow_forwardTernary Phase Diagram+ Material balance One thousand kg/h of a (50-50 wt%) acetone-in-water solution is to be extracted at 25C in a continuous, countercurrent system with pure 1,1,2-trichloroethane to obtain a raffinate containing 10 wt% acetone. Using the following equilibrium data, determine with an equilateral-triangle diagram: a- the minimum flow rate of solvent; b- the number of stages required for a solvent rate equal to 1.5 times minimum, and composition of each streamleaving each stage. c. Repeat the calculation of (a) and (b) if the solvent used has purity 93wt% (4wr% acetone, 3wt% water impurities) acetone water 1,1,2-trichloroethane Raffinate. Weight Extract. Weight 0.6 0.13 0.27 Fraction Acetone Fraction Acetone 0.5 0.04 0.46 0.44 0.56 0.4 0.03 0.57 0.29 0.40 0.3 0.02 0.68 0.12 0.18 0.2 0.015 0.785 0.0 0.0 0.1 0.01 0.89 0.55 0.35 0.1 0.5 0.43 0.07 0.4 0.57 0.03 0.3 0.68 0.02 0.2 0.79 0.01 0.1 0.895 0.005arrow_forwardI need a detailed drawing with explanation so A 4 しか شكا Write a complete C++ program that includes a function to check whether a given sequence of parentheses is balanced using stack or Linked stack 7:30 م PU + 9625 =-2c125 750 x2.01 58³arrow_forward
- Ternary Phase Diagram+ Material balance Feed mixture weighing 200 kg of unknown composition containing water, acetic acid, isopropyl ether is contacted in a single stage with 280kg mixture containing 40wt% acetic acid. 10wt% water and 50wt% isopropyl ether. The resulting raffinate layer weight 320 kg and containing 29.5 wt% acetic acid, 66.5 wt% water and 4wt% isopropyl ether. Determine the composition of the original feed mixture and the extract layer Water layer Isopropyl ether layer acetic acid 0 water 98.8 Isopropyl ether 1.2 acetic acid 0 water Isopropyl ether 0.6 99.4 0.69 98.1 1.2 0.18 0.5 99.3 1.41 97.1 1.5 0.37 0.7 98.9 2.89 95.5 1.6 0.79 0.8 98.4 6.42 91.7 1.9 1.93 1 97.1 13.3 84.4 2.3 4.82 1.9 93.3 25.5 71.1 3.4 11.4 3.9 84.7 36.7 58.9 4.4 21.6 6.9 71.5 44.3 5.1 10.6 31.1 10.8 58.5 46.4 37.1 16.5 36.2 15.1 48.7arrow_forwardGraphically+Material balance 2500 kg/hr of (20-80) nicotine water solution is to be extracted with benzene containing 0.5% nicotine in the 1st and 2nd stages while the 3rd stage is free of nicotine. Cross-current operation is used with different amounts of solvent for each stages 2000kg/hr in the 1st stage, 2300 kg/hr in the 2nd stage, 2600 kg/hr in the 3rd, determine:- a- The final raffinate concentration and % extraction. b- b-The minimum amount of solvent required for counter-current operation if the minimum concentration will be reduced to 5% in the outlet raffinate. Equilibrium data Wt % Nicotine in water 0 4 16 25 Wt % Nicotine in benzene 0 4 21 30arrow_forwardgraphically +material balance 1000 Kg/hr on an acetone water mixture containing 10% of acetone is to be extracted with trichloroethane. The recovered solvent to be used is free of acetone. If 95% recovery of acetone is desired, the equilibrium relationship is given by kg acetone/kg trichloroethane 1.65 kg acetone/kg water. Estimate the number of stages required if 1.5 times the minimum solvent is used when: - a- b- Cross-current is to be extracted. b- Counter-current is to be extracted.arrow_forward
- use Graphically 1000 Kg/hr on an acetone water mixture containing 10% of acetone is to be extracted with trichloroethane. The recovered solvent to be used is free of acetone. If 95% recovery of acetone is desired, the equilibrium relationship is given by kg acetone/kg trichloroethane 1.65 kg acetone/kg water. Estimate the number of stages required if 1.5 times the minimum solvent is used when: - Cross-current is to be extracted. a- b- b- Counter-current is to be extracted.arrow_forwardA solution of 5% acetaldehyde in toluene is to be extracted with water in five stage co-current operation. If 25kg/100kg feed is used, what is the mass of acetaldehyde extracted and the final concentration? The Equilibrium relation is given by: kg acetaldehyde /kg water = 2.2 kg acetaldehyde / kg toluene.arrow_forwardFeed mixture weighing 200 kg of unknown composition containing water, acetic acid, isopropyl ether is contacted in a single stage with 280kg mixture containing 40wt% acetic acid. 10wt% water and 50wt% isopropyl ether. The resulting raffinate layer weight 320 kg and containing 29.5wt% acetic acid, 66.5 wt% water and 4wt% isopropyl ether. Determine the composition of the original feed mixture and the extract layer Water layer Isopropyl ether layer acetic acid water Isopropyl ether acetic acid water Isopropyl ether 0 98.8 1.2 0 0.6 99.4 0.69 98.1 1.2 0.18 0.5 99.3 1.41 97.1 1.5 0.37 0.7 98.9 2.89 95.5 1.6 0.79 0.8 98.4 6.42 91.7 1.9 1.93 1 97.1 13.3 84.4 2.3 4.82 1.9 93.3 25.5 71.1 3.4 11.4 3.9 84.7 36.7 58.9 4.4 21.6 6.9 71.5 44.3 5.1 10.6 31.1 10.8 58.5 46.4 37.1 16.5 36.2 15.1 48.7arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





