
Concept explainers
(a)
Interpretation:
The empirical formula of the given hydrocarbon has to be determined.
Concept Introduction:
The hydrocarbon compounds are compounds which contains only carbon and hydrogen atoms. The combustion of hydrocarbon compounds produces large amount of heat. Combustion reaction of a hydrocarbon results in the formation of carbon dioxide and water.
(a)
Answer to Problem 101QRT
The empirical formula of given hydrocarbon is
Explanation of Solution
The mass of carbon dioxide produced is
The mass of water produced is
The molar mass of carbon dioxide is
The molar mass of water is
Use the expression to calculate number of moles.
Substitute
Therefore, the number of moles of carbon dioxide is
Consider the combustion reaction of hydrocarbon as follows.
Here,
When combustion of hydrocarbon takes place, the carbon atoms present in hydrocarbon gets converted to carbon dioxide molecule. One molecule of carbon dioxide contains one carbon atom. Therefore, the number of moles of carbon atoms present in hydrocarbon is equal to the moles of carbon dioxide.
Therefore, moles of carbon atom in hydrocarbon is
Substitute
Therefore, number of moles of water is
When the combustion of hydrocarbon takes place the hydrogen atoms present in hydrocarbon gets converted into hydrogen atoms of water molecule. One molecule of water contains two hydrogen atoms. Therefore, the number of moles of hydrogen atom present in hydrocarbon is twice the number of moles of water.
Therefore, the number of moles of hydrogen atoms is
The smallest number of moles is
Use the expression to calculate mole ratio.
Substitute
Substitute
Therefore, the empirical formula of given hydrocarbon is
(b)
Interpretation:
The given hydrocarbon is an
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 101QRT
The given hydrocarbon compound is an alkene.
Explanation of Solution
The general formula of alkane is
(c)
Interpretation:
The Lewis structure for given hydrocarbon has to be drawn.
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 101QRT
The Lewis structure for given hydrocarbon is as follows.
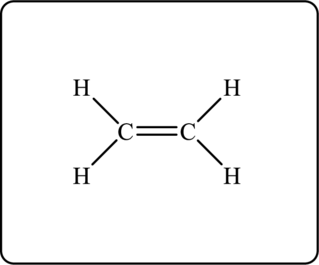
Another possible structure is as follows.
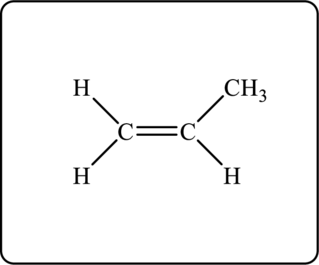
Explanation of Solution
The given hydrocarbon has empirical formula
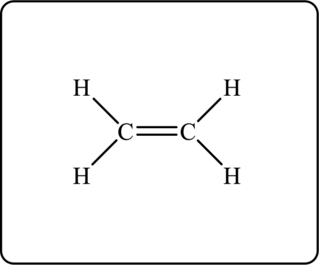
Figure 1
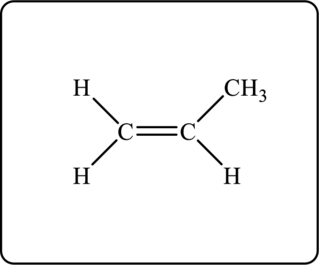
Figure 2
Want to see more full solutions like this?
Chapter 10 Solutions
Chemistry: The Molecular Science
- Identify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forward
- a. H3C CH3 H, 1.0 equiv. Br2arrow_forwardH3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forward
- in the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forwardIf we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

