
Concept explainers
Interpretation: The structure of six
Concept introduction: Alkenes are the compounds in which
Answer to Problem 10.1P
The structure of six alkenes having molecular formula
Explanation of Solution
The given molecular formula of the compound is
The first possible structure of alkene with molecular formula
![]()
Figure 1
The first possible structure of alkene contains five carbon and ten hydrogen atoms. The double bond is present between first and second carbon atom.
The second possible structure of alkene with molecular formula
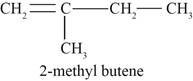
Figure 2
The second possible structure of alkene contains five carbon and ten hydrogen atoms. The double bond is present between first and second carbon atom. One methyl substituent is attached to the second carbon atom.
The third possible structure of alkene with molecular formula
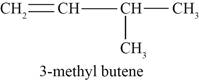
Figure 3
The third possible structure of alkene contains five carbon and ten hydrogen atoms. The double bond is present between first and second carbon atom. One methyl substituent is attached to the third carbon atom.
The fourth possible structure of alkene with molecular formula
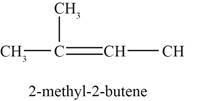
Figure 4
The fourth possible structure of alkene contains five carbon and ten hydrogen atoms. The double bond is present between second and third carbon atom. One methyl substituent is attached to the second carbon atom.
The fifth possible structure of alkene with molecular formula
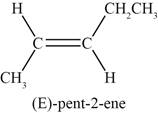
Figure 5
The fifth possible structure of alkene contains five carbon and ten hydrogen atoms. The double bond is present between second and third carbon atom. Substituents attached to the second and third carbon atom are on different side.
The sixth possible structure of alkene with molecular formula
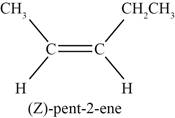
Figure 6
The sixth possible structure of alkene contains five carbon and ten hydrogen atoms. The double bond is present between second and third carbon atom. Substituents attached to the second and third carbon atom are on same side.
A pair of diastereomers exists when two substituents attached to each of the double bonded carbon atoms are different. The fifth and sixth structure indicates that the substituents bonded to the
Therefore, a pair of disatereomers is shown below.
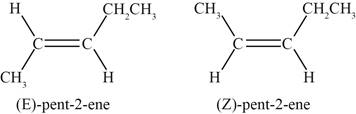
Figure 7
The structure of six alkenes having molecular formula
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry
- Draw the major product of this solvolysis reaction. Ignore any inorganic byproducts. + CH3CH2OH Drawing Q Atoms, Bonds and Rings OCH2CH3 || OEt Charges OH 00-> | Undo Reset | Br Remove Done Drag To Pan +arrow_forwardDraw the major product of this SN1 reaction. Ignore any inorganic byproducts. CH3CO2Na CH3CO2H Drawing + Br Q Atoms, Bonds and Rings OAC Charges OH ОАс Na ဂ Br Undo Reset Remove Done Drag To Pan +arrow_forwardOrganic Functional Groups entifying positions labeled with Greek letters in acids and derivatives 1/5 ssible, replace an H atom on the a carbon of the molecule in the drawing area with a ce an H atom on the ẞ carbon with a hydroxyl group substituent. ne of the substituents can't be added for any reason, just don't add it. If neither substi er the drawing area. O H OH Oneither substituent can be added. Check D 1 Accessibility ado na witharrow_forward
- Differentiate between electrophilic and nucleophilic groups. Give examples.arrow_forwardAn aldehyde/ketone plus an alcohol gives a hemiacetal, and an excess of alcohol gives an acetal. The reaction is an equilibrium; in aldehydes, it's shifted to the right and in ketones, to the left. Explain.arrow_forwardDraw a Haworth projection or a common cyclic form of this monosaccharide: H- -OH H- OH H- -OH CH₂OHarrow_forward
- Answer the question in the first photoarrow_forwardGgggffg2258555426855 please don't use AI Calculate the positions at which the probability of a particle in a one-dimensional box is maximum if the particle is in the fifth energy level and in the eighth energy level.arrow_forwardExplain the concepts of hemiacetal and acetal.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

