
Chemistry for Changing Times
14th Edition
ISBN: 9780134212777
Author: John W. Hill; Terry W. McCreary
Publisher: Pearson Education (US)
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 1, Problem 77AP
Interpretation Introduction
Interpretation: To determine the mass of block in grams.
Concept Introduction:
Mass is the amount of matter contained in a body.
Density is determined by using the formula:
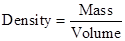
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Name
1) 3-fluoro, 1-butene
2) 2-heptene
2,3-difluoro-
1-pentene
4) 6-iodo,4-methyl-
2-decyne
5) 4,4-dibromo-
1,2-butandiol
Complete structural formula
F
-C=C-C-C-
Line formula
Condensed structural formula
N
F
CH2=CHCHFCH3
1.
Part 1: Naming Organic Compounds
он
H₁C-C-CH3
CH3
Br
CI CI
2. Br-CH-CH-CH₂
H₂C-CH-C= -CH-CH2-CH3
3.
HC-CH-CH-C-OH
5. H₂C-CH-CH₂-OH
7.
OH
4.
CH
CH₂-CH₂
6.
сно
CH-CH-CH-CH₂-CH₂
H₁₂C-CH-CH-CH-CH₁₂-CH₁₂
8.
OH
11
Organic Chemistry
Organic Nomenclature Practice
Name/Functional Group
n-butane
Formula
Structural Formula
(1) C4tt10
H3C
C-
(2) CH3CH2CH2 CH 3
H₂
-CH3
Н2
name & functional group
(1) and (2)
OH
H₁₂C
Н2
name only
(1) and (2)
name only
(1) and (2)
H₁C - = - CH₂
Н2
HC=C-C
CH3
Chapter 1 Solutions
Chemistry for Changing Times
Ch. 1 - Prob. 1RQCh. 1 - Why do experiments have to be done to support a...Ch. 1 - Why can't scientific methods always be used to...Ch. 1 - How does technology differ from science?Ch. 1 - Prob. 5RQCh. 1 - Prob. 6RQCh. 1 - What is a DQ? What does a large DQ mean? Why is it...Ch. 1 - What derived units of (a) mass and (b) length are...Ch. 1 - What is the Sl-derived unit for volume? What...Ch. 1 - Prefix Symbol Definition tera- T 1012 - M - centi-...
Ch. 1 - Prob. 11RQCh. 1 - Identify the following work as either applied...Ch. 1 - Penicillin kills bacteria, thus saving the lives...Ch. 1 - Prob. 14PCh. 1 - Prob. 15PCh. 1 - X-rays are widely used in medicine and dentistry....Ch. 1 - Prob. 17PCh. 1 - The virus called HIV causes AIDS, a devastating...Ch. 1 - Which are realistic masses for a cellular...Ch. 1 - In Europe, A2 sized paper measures 594 mm 420 mm,...Ch. 1 - Which one(s) of the following are likely to be...Ch. 1 - Sample X on the moon has exactly the same mass as...Ch. 1 - Which of the following is a reasonable volume for...Ch. 1 - Which of the following is a reasonable...Ch. 1 - Earth's oceans contain 3.501 0 8 mi3 of water and...Ch. 1 - What is the area of Earth's oceans in square...Ch. 1 - Consider the two tubes shown below. The aluminum...Ch. 1 - Which one(s) of the following could be the inside...Ch. 1 - Identify the following as physical or chemical...Ch. 1 - Identify the following as physical or chemical...Ch. 1 - Identify the following changes as physical or...Ch. 1 - Identify the following changes as physical or...Ch. 1 - Identify each of the following as a substance or a...Ch. 1 - Identify each of the following as a substance or a...Ch. 1 - Which of the following mixtures are homogeneous,...Ch. 1 - Which of the following mixtures are homogeneous,...Ch. 1 - Every sample of the sugar glucose (no matter where...Ch. 1 - An advertisement for shampoo says, "Pure shampoo,...Ch. 1 - Which of the following represent elements, and...Ch. 1 - Prob. 40PCh. 1 - Prob. 41PCh. 1 - Without consulting tables, write a symbol for each...Ch. 1 - In his 1739 textbook, Traite elementaire de...Ch. 1 - In 1774 Joseph Priestley isolated a gas that he...Ch. 1 - Change the unit for each of the following...Ch. 1 - Convert each of the following measurements to the...Ch. 1 - Carry out the following conversions. a. 5.52104 mL...Ch. 1 - Carry out the following conversions. a. 546 mm to...Ch. 1 - Indicate which is the larger unit in each pair. a....Ch. 1 - There are about...Ch. 1 - Express the length of a 31 -cm ruler in (a) mm,...Ch. 1 - What is the volume in liters of (a) a 352-mL soft...Ch. 1 - (You may need data from Table 1.6 for some of...Ch. 1 - (You may need data from Table 1.6 for some of...Ch. 1 - (You may need data from Table 1.6 for some of...Ch. 1 - (You may need data from Table 1.6 for some of...Ch. 1 - (You may need data from Table 1.6 for some of...Ch. 1 - (You may need data from Table 1.6 for some of...Ch. 1 - (You may need data from Table 1.6 for some of...Ch. 1 - (You may need data from Table 1.6 for some of...Ch. 1 - Prob. 61PCh. 1 - (You may need data from Table 1.6 for some of...Ch. 1 - (You may need data from Table 1.6 for some of...Ch. 1 - (You may need data from Table 1.6 for some of...Ch. 1 - Liquid nitrogen, used for freezing sperm samples,...Ch. 1 - Normal body temperature is about 37 °C. What is...Ch. 1 - Prob. 67PCh. 1 - Prob. 68PCh. 1 - A certain chemistry class is 1.00 microcentury ( ...Ch. 1 - 70. A unit of beauty, a helen, thought to have...Ch. 1 - 71. English chemist William Henry studied the...Ch. 1 - Prob. 72APCh. 1 - Prob. 73APCh. 1 - For Problems 74 and 75t, classify each of the...Ch. 1 - For Problems 74 and 75, classify each of the...Ch. 1 - Prob. 76APCh. 1 - Prob. 77APCh. 1 - Prob. 78APCh. 1 - Prob. 79APCh. 1 - Prob. 80APCh. 1 - Prob. 81APCh. 1 - Prob. 82APCh. 1 - Prob. 83APCh. 1 - Prob. 84APCh. 1 - Prob. 85APCh. 1 - Prob. 86APCh. 1 - The density of a planet can be approximated from...Ch. 1 - The extrasolar planet HAT-P-i orbits a star 450...Ch. 1 - Prob. 89APCh. 1 - Prob. 90APCh. 1 - Prob. 91APCh. 1 - Prob. 92APCh. 1 - Prob. 1.1CTECh. 1 - Prob. 1.2CTECh. 1 - Prob. 1.3CTECh. 1 - Prob. 1.4CTECh. 1 - Prob. 1.5CTECh. 1 - Prob. 1.6CTECh. 1 - Prob. 1CGPCh. 1 - Prob. 2CGPCh. 1 - Prob. 3CGPCh. 1 - Materials Needed: • 1/4 cup dark corn syrup • 1/4...Ch. 1 - Materials Needed: • 1/4 cup dark corn syrup • 1/4...Ch. 1 - Prob. 3CHQCh. 1 - Prob. 4CHQCh. 1 - Materials Needed: • 1/4 cup dark corn syrup • 1/4...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Under aqueous basic conditions, nitriles will react to form a neutral organic intermediate 1 that has an N atom in it first, and then they will continue to react to form the final product 2: NC H₂O он- H₂O 1 2 OH Draw the missing intermediate 1 and the final product 2 in the box below. You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardAssign these COSY Spectrumarrow_forwardAssign these C-NMR and H-NMR Spectrumarrow_forward
- Predict the product of this organic reaction: IZ + HO i P+H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click and drag to start drawing a structure. ☐ :arrow_forwardPredict the products of this organic reaction: 0 O ----- A + KOH ? CH3-CH2-C-O-CH2-C-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. X ⑤ èarrow_forwardPredict the products of this organic reaction: O CH3 + H2O + HCI A A? CH3-CH2-C-N-CH3 Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward
- What is the missing reactant in this organic reaction? R+ HO-C-CH2-CH3 0= CH3 CH3 —CH, C−NH—CH CH3 + H₂O Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. No Answer Click anywhere to draw the first atom of your structure. €arrow_forward个 CHEM&131 9267 - $25 - Intro to Mail - Hutchison, Allison (Student x Aktiv Learnin https://app.aktiv.com Draw the product of the reaction shown below. Ignore inorganic byproducts. + Na2Cr2O7 Acetone, H2SO4 Type here to search Dryng OH W Prarrow_forwardPredict the products of this organic reaction: OH + NaOH A? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click and drag to start drawing a structure. ✓ Sarrow_forward
- Predict the products of this organic reaction: CH3-C-O-CH2-CH2-C-CH3 + H₂O ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No reaction Click anywhere to draw the first atom of your structure. :☐ darrow_forwardDE d. Draw an arrow pushing mechanism for the following IN O CI N fo 人 P Polle DELL prt sc home end ins F5 F6 F7 F8 F9 F10 F11 F12arrow_forwardPredict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY