
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 60P
Rank the following in order of decreasing acidity. Although none of these specific
structures appear in Table
reasoning.
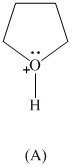
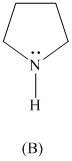
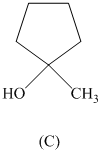
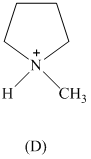
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
Chapter 1 Solutions
Organic Chemistry - Standalone book
Ch. 1.1 - How many electrons does carbon have? How many are...Ch. 1.1 - Referring to the periodic table as needed, write...Ch. 1.2 - Species that have the same number of electrons are...Ch. 1.2 - Which of the following ions possess a noble gas...Ch. 1.2 - Prob. 5PCh. 1.3 - Prob. 6PCh. 1.3 - Problem 1.7 All of the hydrogens are bonded to...Ch. 1.4 - Problem 1.8 In which of the compounds...Ch. 1.4 - Indicate the direction of the dipole for the...Ch. 1.5 - Prob. 10P
Ch. 1.5 - The following inorganic species will be...Ch. 1.5 - Prob. 12PCh. 1.6 - Prob. 13PCh. 1.6 - Problem 1.14 Nitrosomethane and formaldoxime both...Ch. 1.6 - Prob. 15PCh. 1.7 - All of the bonds in the carbonate ion (CO32-) are...Ch. 1.7 - Prob. 17PCh. 1.8 - Prob. 18PCh. 1.8 - Prob. 19PCh. 1.9 - Sodium borohydride, NaBH4, has an ionic bond...Ch. 1.9 - Prob. 21PCh. 1.10 - Which of the following compounds would you expect...Ch. 1.11 - Using the curved arrow to guide your reasoning,...Ch. 1.11 - Prob. 24PCh. 1.11 - Prob. 25PCh. 1.12 - Prob. 26PCh. 1.12 - Prob. 27PCh. 1.12 - Prob. 28PCh. 1.12 - Prob. 29PCh. 1.12 - Prob. 30PCh. 1.13 - Which is the stronger acid, H2O or H2S? Which is...Ch. 1.13 - Prob. 32PCh. 1.13 - Prob. 33PCh. 1.13 - Hypochlorous and hypobromous acid (HOClandHOBr)...Ch. 1.13 - Prob. 35PCh. 1.13 - Prob. 36PCh. 1.14 - What is the equilibrium constant for the following...Ch. 1.14 - Prob. 38PCh. 1.14 - Prob. 39PCh. 1.15 - Write an equation for the Lewis acid/Lewis base...Ch. 1 - Write a Lewis formula for each of the following...Ch. 1 - Prob. 42PCh. 1 - Write structural formulas for all the...Ch. 1 - Prob. 44PCh. 1 - Expand the following structural representations so...Ch. 1 - Each of the following species will be encountered...Ch. 1 - Consider Lewis formulas A, B, and C: H2 C -NN:...Ch. 1 - Prob. 48PCh. 1 - Prob. 49PCh. 1 - Prob. 50PCh. 1 - Prob. 51PCh. 1 - Prob. 52PCh. 1 - Prob. 53PCh. 1 - Prob. 54PCh. 1 - Which compound in each of the following pairs...Ch. 1 - With a pKa of 11.6, hydrogen peroxide is a...Ch. 1 - The structure of montelukast, an antiasthma drug,...Ch. 1 - One acid has a pKa of 2, the other has a pKa of 8....Ch. 1 - Calculate Ka for each of the following acids,...Ch. 1 - Rank the following in order of decreasing acidity....Ch. 1 - Rank the following in order of decreasing...Ch. 1 - Consider 1.0 M aqueous solutions of each of the...Ch. 1 - Prob. 63PCh. 1 - Prob. 64PCh. 1 - Prob. 65PCh. 1 - Prob. 66PCh. 1 - Prob. 67PCh. 1 - Prob. 68PCh. 1 - Amide Lewis Structural Formulas Lewis formulas are...Ch. 1 - Amide Lewis Structural Formulas Lewis formulas are...Ch. 1 - Amide Lewis Structural Formulas Lewis formulas are...Ch. 1 - Prob. 72DSPCh. 1 - Amide Lewis Structural Formulas Lewis formulas are...Ch. 1 - Amide Lewis Structural Formulas Lewis formulas are...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY