
Concept explainers
Interpretation: The location of ozone in the Earth’s atmosphere needs to be explained.
Concept introduction: The layer/film of gases around our Earth is defined as an atmosphere. Life on Earth is impossible without it. On the basis of temperature, the atmosphere comprises four main layers which are mentioned below:
- Troposphere
- Stratosphere
- Mesosphere
- Thermosphere
Answer to Problem 26A
The ozone layer is naturally formed by a reactive process in the stratosphere. The ozone layer is important because it protects our Earth from UV lights.
Explanation of Solution
90 % of ozone is located in the stratosphere. Above Earth's surface, the stratosphere about 10 to 16 kilometers long (on altitude). Excluding this, the stratosphere extends up to 50 kilometers (nearby). The ozone layer is naturally formed (or produced) in the stratosphere. The following image will help you to locate ozone in Earth’s atmosphere:
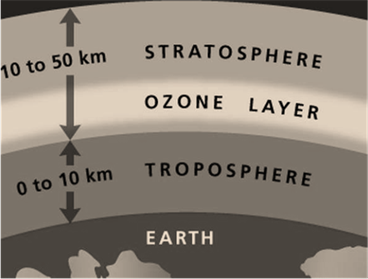
In the stratosphere, formation(by the reactive process) of the ozone layer is mentioned below:
Step 1: Solar ultraviolet radiation (or sunlight) breaks apart oxygen (O) molecule to form 2 separate oxygen atoms.
Step 2: Individually, the atom later undergoes a binding collision with another present oxygen molecule. This process forms an ozone molecule.
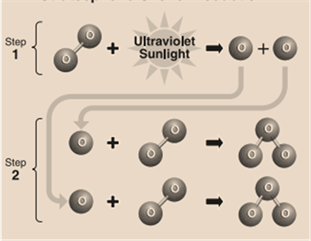
Step 3: After this process, 3 oxygen molecules plus sunlight react together to form 2 ozone molecules. This becomes the ozone layer. The reaction of this process is mentioned below:
The above reaction takes place in the presence of sunlight.
The ozone layer is a ring of protective gases which protects Earth from UV light.
Chapter 1 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Campbell Biology: Concepts & Connections (9th Edition)
Microbiology: An Introduction
Campbell Essential Biology (7th Edition)
Microbiology: An Introduction
Campbell Biology in Focus (2nd Edition)
Campbell Biology (11th Edition)
- Macmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. A C6H5 CH3arrow_forwardProvide the reagents for the following reactions.arrow_forward
- If I have 1-bromopropene, to obtain compound Z, I have to add two compounds A1 and A2. Indicate which compounds are needed. P(C6H5)3arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forward
- Predict the major organic product(s) for the following reactions.arrow_forwardProvide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forward
- If I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forwardIf I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





