
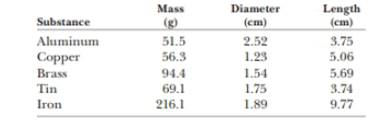
The data in the following table represent measurements of the masses and dimensions of solid cylinders of aluminum, copper, brass, tin, and iron. (a) Use these data to calculate the densities of these substances. (b) State how your results compare with those given in Table 14.1.
(a)
The densities of each substance.
Answer to Problem 1.61AP
The density of aluminum solid cylinders is
Explanation of Solution
Given info: The mass, diameter and length of each substance are given below,
| Substance | Mass
| Diameter
| Length
|
| Aluminum |
|
|
|
| Copper |
|
|
|
| Brass |
|
|
|
| Tin |
|
|
|
| Iron |
|
|
|
Formula to calculate the density of substance is,
Here,
Write the expression for the volume of solid cylinder,
Here,
Substitute
For aluminum:
Substitute
Thus, the density of aluminum solid cylinders is
For copper:
Substitute
Thus, the density of copper solid cylinders is
For brass:
Substitute
Thus, the density of brass solid cylinders is
For tin:
Substitute
Thus, the density of tin solid cylinders is
For iron:
Substitute
Thus, the density of iron solid cylinders is
Conclusion:
Therefore, the density of aluminum solid cylinders is
(b)
The comparison between results of part (a) and table
Answer to Problem 1.61AP
The density of aluminum from table is
Explanation of Solution
Given info:
Formula to calculate the percentage error is,
Here,
For aluminum:
From part (a), the density of the aluminum is
Substitute
Thus, the density of aluminum from table is
For copper:
From part (a), the density of the copper is
Substitute
Thus, the density of copper from table is
For brass:
From part (a), the density of the brass is
Substitute
Thus, the density of brass from table is
For tin:
From part (a), the density of the tin is
Substitute
Thus, the density of tin from table is
For iron:
From part (a), the density of the iron is
Substitute
Thus, the density of iron from table is
Conclusion:
Therefore, the density of aluminum from table is
Want to see more full solutions like this?
Chapter 1 Solutions
EBK PHYSICS FOR SCIENTISTS AND ENGINEER
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
Loose Leaf For Integrated Principles Of Zoology
Chemistry & Chemical Reactivity
Biology: Life on Earth (11th Edition)
Fundamentals Of Thermodynamics
Fundamentals of Anatomy & Physiology (11th Edition)
- Show that the units 1 v2/Q = 1 W, as implied by the equation P = V²/R. Starting with the equation P = V²/R, we can get an expression for a watt in terms of voltage and resistance. The units for voltage, V, are equivalent to [? v2 v2 A, are equivalent to J/C ✓ X . Therefore, 1 = 1 = 1 A V1 J/s Ω V-A X = 1 W. . The units for resistance, Q, are equivalent to ? The units for current,arrow_forwardPlease solve and answer the question correctly please. Thank you!!arrow_forwardPlease solve and answer the question correctly please. Thank you!!arrow_forward
- According to the provided information answer the question accorrding to grade 11 physics Jerry has decided to give up his part-time job for a new career, cat-burglar! Jerry loves the idea of dressing up like a cat all day and of course the chance of meeting Cat Woman! On Jerry's first "job" he figures out his escape plan. He travels 3.0 km south for 15 minutes and then 8.0 km west for 1.5 hours before reaching his house. Draw a sketch diagram of the path he took with all the appropriate labels.arrow_forwardPlease solve and answer all parts of the question correctly please. Thank you!!arrow_forwardPlease solve and answer this question correctly please. Thank you!!arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College





