
Concept explainers
(a)
Interpretation:
The Lewis structure of the
Concept introduction:
Lewis structure of a molecule or ion is drawn considering only the valence electrons of the atoms. In case of ions, for each negative charge an electron is added while for each positive charge an electron is subtracted on the ion. Each bond shows a shared pair of electrons. Single, double, and triple bonds are represented by one, two, and three lines, respectively, connecting the two atoms. Non-bonding electrons are shown as dots, generally as lone pairs. Atoms in a Lewis structure should not exceed their valency. The atoms from the 2nd row must not exceed an octet of valence electrons. Atoms from the 3rd row onward may exceed an octet by up to four electrons if they are central atoms.
The formal charge (FC) on an atom is determined as the difference between its group number and the actual number of electrons it possesses in the molecule or ion. The formula to calculate formal charge is as below.
Answer to Problem 1.50P
The Lewis structure of the
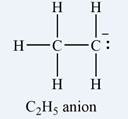
The atom that bears a non-zero formal charge is the carbon atom on the right, bonded to only two hydrogens.
Explanation of Solution
The total of valence electrons based on the formula
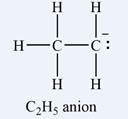
The formal charge on each hydrogen atom is zero:
The formal charge on the first carbon (on the left) is zero:
The formal charge on the second carbon is
Therefore, it is the carbon atom on the right, bearing only two hydrogen atoms, that has a non-zero formal charge.
The formal charge on an atom is the difference in its group number and the actual number of electrons it possesses in the molecule or ion.
(b)
Interpretation:
The Lewis structure of the
Concept introduction:
Lewis structure of a molecule or ion is drawn considering only the valence electrons of the atoms. Each bond shows a shared pair of electrons. Single, double, and triple bonds are represented by one, two, and three lines, respectively, connecting the two atoms. Non-bonding electrons are shown as dots, generally as lone pairs. Atoms in a Lewis structure should not exceed their valency. The atoms from the 2nd row must not exceed an octet of valence electrons. Atoms from the 3rd row onward may exceed an octet by up to four electrons if they are central atoms.
The formal charge on an atom is determined as the difference between its group number and the actual number of electrons it possesses in the molecule or ion. The formula to calculate formal charge is as below.
Answer to Problem 1.50P
The Lewis structure of the
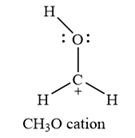
The carbon atom is the one that has a non-zero formal charge.
Explanation of Solution
Carbon is the central atom in this cation, with two hydrogen atoms and the oxygen atom bonded to it. The third hydrogen atom is bonded to the oxygen as shown below:
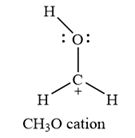
The formal charges on the atoms are calculated as below:
Hydrogen atoms:
Carbon atom:
Oxygen atom:
An alternate structure can be drawn with all three hydrogen atoms and the oxygen atom bonded to the central carbon. However, this will put the positive charge on a highly electronegative oxygen, making it unstable. Therefore, the above mentioned structure is the most likely structure.
Therefore, the carbon atom has a non-zero formal charge.
The formal charge on an atom is the difference in its group number and the actual number of electrons it possesses in the molecule or ion.
(c)
Interpretation:
The Lewis structure of the
Concept introduction:
Lewis structure of a molecule or ion is drawn considering only the valence electrons of the atoms. Each bond shows a shared pair of electrons. Single, double, and triple bonds are represented by one, two, and three lines, respectively, connecting the two atoms. Non-bonding electrons are shown as dots, generally as lone pairs. Atoms in a Lewis structure should not exceed their valency. The atoms from the 2nd row must not exceed an octet of valence electrons. Atoms from the 3rd row onward may exceed an octet by up to four electrons if they are central atoms.
The formal charge on an atom is determined as the difference between its group number and the actual number of electrons it possesses in the molecule or ion. The formula to calculate formal charge is as below.
Answer to Problem 1.50P
The Lewis structure of the
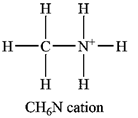
The atom with a non-zero formal charge is the nitrogen.
Explanation of Solution
Carbon usually forms four bonds; therefore, it is a central atom. Three of the hydrogen atoms and the nitrogen atom must be bonded to it. The nitrogen atom in turn is also a central atom, with the remaining three hydrogen atoms bonded to it. Normally nitrogen forms only three bonds, but it can use its lone pair to form another bond, generally to a hydrogen ion. Therefore, the Lewis structure of the cation must be

The formal charges on the atoms are calculated as follows:
Hydrogen atoms:
Carbon atom:
Nitrogen atom:
Therefore, it is the nitrogen atom that has a non-zero formal charge.
The formal charge on an atom is the difference in its group number and the actual number of electrons it possesses in the molecule or ion.
(d)
Interpretation:
The Lewis structure of the
Concept introduction:
Lewis structure of a molecule or ion is drawn considering only the valence electrons of the atoms. Each bond shows a shared pair of electrons. Single, double, and triple bonds are represented by one, two, and three lines, respectively, connecting the two atoms. Non-bonding electrons are shown as dots, generally as lone pairs. Atoms in a Lewis structure should not exceed their valency. The atoms from the 2nd row must not exceed an octet of valence electrons. Atoms from the 3rd row onward may exceed an octet by up to four electrons if they are central atoms.
The formal charge on an atom is determined as the difference between its group number and the actual number of electrons it possesses in the molecule or ion. The formula to calculate formal charge is as below.
Answer to Problem 1.50P
The Lewis structure of
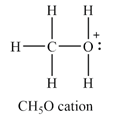
The atom with non-zero formal charge is the oxygen atom.
Explanation of Solution
The

The formal charges on the atoms are calculated as follows:
Hydrogen atoms:
Carbon atom:
Oxygen atom:
Therefore, the oxygen has a non-zero formal charge.
The formal charge on an atom is the difference in its group number and the actual number of electrons it possesses in the molecule or ion.
(e)
Interpretation:
The Lewis structure of the
Concept introduction:
Lewis structure of a molecule or ion is drawn considering only the valence electrons of the atoms. Each bond shows a shared pair of electrons. Single, double, and triple bonds are represented by one, two, and three lines, respectively, connecting the two atoms. Non-bonding electrons are shown as dots, generally as lone pairs. Atoms in a Lewis structure should not exceed their valency. The atoms from the 2nd row must not exceed an octet of valence electrons. Atoms from the 3rd row onward may exceed an octet by up to four electrons if they are central atoms.
The formal charge on an atom is determined as the difference between its group number and the actual number of electrons it possesses in the molecule or ion. The formula to calculate formal charge is as below.
Answer to Problem 1.50P
The Lewis structure of the
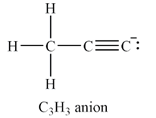
The carbon atom at the right end of the chain has a non-zero formal charge.
Explanation of Solution
The total of the valence electrons for this anion is
The carbon atoms have capacity to form up to four bonds. If all three hydrogen atoms are on the same carbon, this carbon must be at the end of a chain of three carbon atoms. The other two carbon atoms must be triply bonded to each other in order to complete their octets. The carbon atom at the other end of the chain must have a lone pair so that all the valence electrons are accounted for.
The Lewis structure of the

The formal charges on the atoms are calculated as follows:
Hydrogen atoms:
Carbon atom on the left and in the center of the chain:
Carbon atom at the right end of the chain:
Therefore, it is the carbon atom at the right end of the chain that has a non-zero formal charge.
The formal charge on an atom is the difference in its group number and the actual number of electrons it possesses in the molecule or ion.
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry: Principles And Mechanisms
- Q5: Label each chiral carbon in the following molecules as R or S. Make sure the stereocenter to which each of your R/S assignments belong is perfectly clear to the grader. (8pts) R OCH 3 CI H S 2pts for each R/S HO R H !!! I OH CI HN CI R Harrow_forwardCalculate the proton and carbon chemical shifts for this structurearrow_forwardA. B. b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion and B. is a neutral molecule. One of these molecules is a highly reactive compound first characterized in frozen noble gas matrices, that self-reacts rapidly at temperatures above liquid nitrogen temperature. The other compound was isolated at room temperature in the early 1960s, and is a stable ligand used in organometallic chemistry. Which molecule is the more stable molecule, and why?arrow_forward
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning



