
Concept explainers
(a)
Interpretation:
For the given species, the complete Lewis structure is to be completed by adding multiple bonds and/or lone pairs.
Concept introduction:
In order to draw a Lewis structure for a molecule, start by counting the total number of valence electrons in a molecule. The number of valence electrons by each atom is the same as its group number. For the given skeleton of the molecule, distribute the remaining electrons as lone pairs. In doing so, start with the outer atoms and work inwards. Try to achieve an octet on each atom other than hydrogen. If there is an atom with less than an octet, increase the atom’s share of electrons by converting lone pairs from neighboring atoms to bonding pairs thereby creating double or triple bonds. For an uncharged atom, carbon atoms will have a maximum of four bonds; Nitrogen will have three bonds and one lone pair, while oxygen will have two bonds and two lone pairs. Hydrogen always contributes to one bond. The number of bonds in case of halogen is one; while there will be three lone pair of electrons on halide atoms.
Answer to Problem 1.24P
The complete Lewis structure for the given species is:
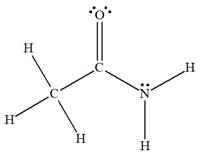
Explanation of Solution
The given species is:
The formula for the species above is
The carbon atom on the left side has four bonds, thus, its octet is complete. The carbon atom in the middle has four bonds, hence, its octet is also complete. The nitrogen atom has three bonds, thus, its octet is not complete. There should be one lone pair of electron on nitrogen. The double bonded oxygen atom has got two bonds. Thus, in order to complete its octet, it should possess two lone pair of electrons. Thus, all the
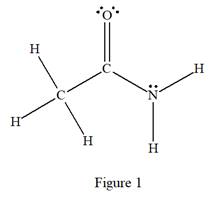
The complete Lewis structure for the given species including multiple bonds and lone pairs is shown in Figure 1 above.
(b)
Interpretation:
For given species, the complete Lewis structure is to be completed by adding multiple bonds and/or lone pairs.
Concept introduction:
In order to draw a Lewis structure for a molecule, start by counting the total number of valence electrons in a molecule. The number of valence electrons by each atom is the same as its group number. For a charged species, each negative charge increases the number of valence electrons by one while each positive charge decreases the number of valence electrons by one. For the given skeleton of the molecule, distribute the remaining electrons as lone pairs. In doing so, start with the outer atoms and work inwards. Try to achieve an octet on each atom other than hydrogen. If there is an atom with less than an octet, increase the atom’s share of electrons by converting lone pairs from neighboring atoms to bonding pairs thereby creating double or triple bonds. For an uncharged atom, carbon atoms will have maximum of four bonds. Nitrogen will have three bonds and one lone pair, while oxygen will have two bonds and two lone pairs. Hydrogen always contributes to one bond. The number of bond in case of halogen is one, while there will be three lone pair of electrons on halide atoms.
Answer to Problem 1.24P
The complete Lewis structure for the given species is:
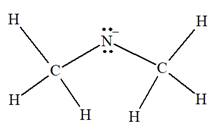
Explanation of Solution
The given species is:
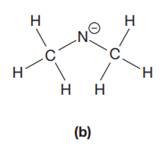
The formula for the species above is
The carbon atom on the left as well as on the right has four bonds, thus, their octets are complete. The nitrogen atom has two bonds and a negative formal charge. This suggests that the remaining four electrons should be present on the nitrogen atom so as to complete its octet and have a negative formal charge. Thus, the complete Lewis structure for the given species is:
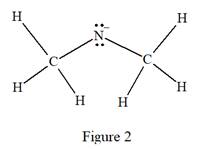
The complete Lewis structure for the given species including multiple bonds and lone pairs is shown in Figure 2 above.
(c)
Interpretation:
For given species, the complete Lewis structure is to be completed by adding multiple bonds and/or lone pairs.
Concept introduction:
In order to draw a Lewis structure for a molecule, start by counting the total number of valence electrons in a molecule. The number of valence electrons by each atom is the same as its group number. For a charged species, each negative charge increase the number of valence electrons by one while each positive charge decrease the number of valence electrons by one. For the given skeleton of the molecule, distribute the remaining electrons as lone pairs. In doing so, start with the outer atoms and work inwards. Try to achieve an octet on each atom other than hydrogen. If there is an atom with less than an octet, increase the atom’s share of electrons by converting lone pairs from neighboring atoms to bonding pairs thereby creating double or triple bonds. For an uncharged atom, carbon atoms will have maximum of four bonds. Nitrogen will have three bonds and one lone pair, while oxygen will have two bonds and two lone pairs. Hydrogen always contributes to one bond. The number of bond in case of halogen is one, while there will be three lone pair of electrons on halide atoms.
Answer to Problem 1.24P
The complete Lewis structure for the given species is:
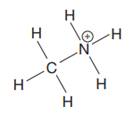
Explanation of Solution
The given species is:
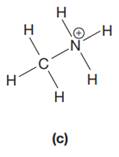
The formula for the species above is
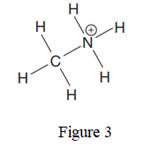
The complete Lewis structure for the given species including multiple bonds and lone pairs is shown in Figure 2 above.
(d)
Interpretation:
For given species, the complete Lewis structure is to be completed by adding multiple bonds and/or lone pairs.
Concept introduction:
In order to draw a Lewis structure for a molecule, start by counting the total number of valence electrons in a molecule. The number of valence electrons by each atom is the same as its group number. For a charged species, each negative charge increase the number of valence electrons by one while each positive charge decrease the number of valence electrons by one. For the given skeleton of the molecule, distribute the remaining electrons as lone pairs. In doing so, start with the outer atoms and work inwards. Try to achieve an octet on each atom other than hydrogen. If there is an atom with less than an octet, increase the atom’s share of electrons by converting lone pairs from neighboring atoms into bonding pairs thereby creating double or triple bonds. For an uncharged atom, carbon atoms will have maximum of four bonds. Nitrogen will have three bonds and one lone pair, while oxygen will have two bonds and two lone pairs. Hydrogen always contributes to one bond. The number of bond in case of halogen is one, while there will be three lone pair of electrons on halide atoms.
Answer to Problem 1.24P
The complete Lewis structure for the given species is:
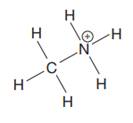
Explanation of Solution
The given species is:
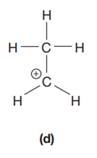
The formula for the species above is
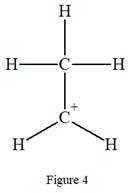
The complete Lewis structure for the given species including multiple bonds and lone pairs is shown in Figure 4 above.
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry: Principles And Mechanisms
- Where are the chiral centers in this molecule? Also is this compound meso yes or no?arrow_forwardA mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forward
- A mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forwardHow many chiral carbons are in the molecule? Farrow_forwardcan someone give the curly arrow mechanism for this reaction written with every intermediate and all the side products pleasearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

