
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
2nd Edition
ISBN: 9780077633707
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.34UKC
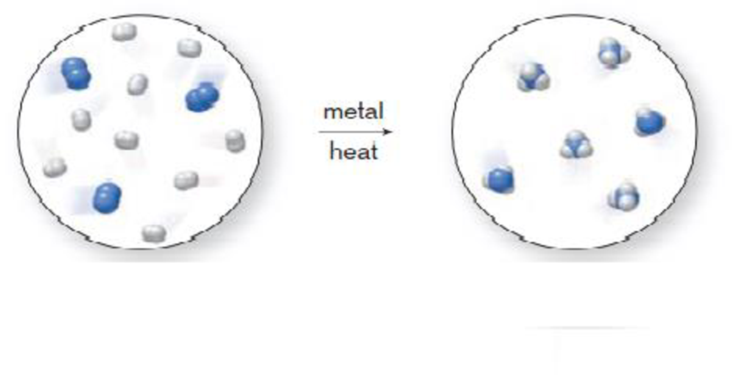
The inexpensive preparation of nitrogen-containing fertilizers begins with mixing together two elements, hydrogen and nitrogen, at high temperature and pressure in the presence of a metal. Does the molecular art depicted below indicate that a chemical or physical change occurs under these conditions? Explain your choice.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Connect 1-Semester Online Access for Principles of General, Organic & Biochemistry
Ch. 1.1 - Imagine that your job as a healthcare professional...Ch. 1.2 - Characterize each process as a physical change or...Ch. 1.2 - Does the molecular art represent a chemical change...Ch. 1.3 - Classify each example of molecular art as a pure...Ch. 1.3 - Prob. 1.5PCh. 1.3 - Classify each item as an element or a compound:...Ch. 1.4 - Prob. 1.7PCh. 1.4 - If a nanometer is one billionth of a meter (0.000...Ch. 1.4 - Prob. 1.9PCh. 1.4 - Prob. 1.10P
Ch. 1.5 - How many significant figures does each number...Ch. 1.5 - Indicate whether each zero in the following...Ch. 1.5 - Prob. 1.13PCh. 1.5 - Carry out each calculation and give the answer...Ch. 1.5 - Prob. 1.15PCh. 1.6 - Prob. 1.16PCh. 1.6 - Prob. 1.17PCh. 1.6 - Prob. 1.18PCh. 1.7 - Prob. 1.19PCh. 1.7 - Prob. 1.20PCh. 1.7 - Prob. 1.21PCh. 1.7 - Carry out each of the following conversions. a....Ch. 1.8 - Prob. 1.23PCh. 1.8 - A patient is prescribed 0.100 mg of a drug that is...Ch. 1.8 - Prob. 1.25PCh. 1.9 - Prob. 1.26PCh. 1.9 - Prob. 1.27PCh. 1.10 - How does the mass of liquid A in cylinder [1]...Ch. 1.10 - Prob. 1.29PCh. 1.10 - Prob. 1.30PCh. 1 - Classify each example of molecular art as a pure...Ch. 1 - (a) Which representation(s) in Problem 1.31...Ch. 1 - When a chunk of dry ice (solid carbon dioxide) is...Ch. 1 - The inexpensive preparation of nitrogen-containing...Ch. 1 - a. What is the temperature on the given Fahrenheit...Ch. 1 - (a) What is the length of the given crayon in...Ch. 1 - Prob. 1.37UKCCh. 1 - Prob. 1.38UKCCh. 1 - Prob. 1.39UKCCh. 1 - Red light has a wavelength of 683 nm. Convert this...Ch. 1 - Prob. 1.41UKCCh. 1 - Prob. 1.42UKCCh. 1 - Prob. 1.43UKCCh. 1 - Prob. 1.44UKCCh. 1 - Label each component in the molecular art as an...Ch. 1 - Label each component in the molecular art as an...Ch. 1 - Describe solids, liquids, and gases in terms of...Ch. 1 - Prob. 1.48APCh. 1 - Prob. 1.49APCh. 1 - Classify each process as a chemical or physical...Ch. 1 - Which quantity in each pair is larger? a. 5 mL or...Ch. 1 - Which quantity in each pair is larger? a. 10 km or...Ch. 1 - Label each quantity as an exact or inexact number....Ch. 1 - Rank the quantities in each group from smallest to...Ch. 1 - How many significant figures does each number...Ch. 1 - Prob. 1.56APCh. 1 - Round each number to three significant figures. a....Ch. 1 - Prob. 1.58APCh. 1 - Prob. 1.59APCh. 1 - Prob. 1.60APCh. 1 - Prob. 1.61APCh. 1 - Prob. 1.62APCh. 1 - Prob. 1.63APCh. 1 - Prob. 1.64APCh. 1 - Prob. 1.65APCh. 1 - Rank the numbers in each group from smallest to...Ch. 1 - Write the recommended daily intake of each...Ch. 1 - Prob. 1.68APCh. 1 - Prob. 1.69APCh. 1 - Carry out each of the following conversions. a. 25...Ch. 1 - Prob. 1.71APCh. 1 - Prob. 1.72APCh. 1 - Prob. 1.73APCh. 1 - Prob. 1.74APCh. 1 - Prob. 1.75APCh. 1 - Prob. 1.76APCh. 1 - Prob. 1.77APCh. 1 - Prob. 1.78APCh. 1 - Prob. 1.79APCh. 1 - Prob. 1.80APCh. 1 - Prob. 1.81APCh. 1 - Prob. 1.82APCh. 1 - Which is the upper layer when each of the...Ch. 1 - Prob. 1.84APCh. 1 - A lab test showed an individuals cholesterol level...Ch. 1 - Prob. 1.86APCh. 1 - Liposuction is a cosmetic procedure used to remove...Ch. 1 - Prob. 1.88APCh. 1 - Prob. 1.89APCh. 1 - Prob. 1.90APCh. 1 - Prob. 1.91APCh. 1 - Prob. 1.92APCh. 1 - Prob. 1.93CPCh. 1 - Prob. 1.94CPCh. 1 - Prob. 1.95CPCh. 1 - Prob. 1.96CPCh. 1 - A soccer player weighed 70.7 kg before a match,...Ch. 1 - Prob. 1.98CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You receive a mixture of table salt and sand and have to separate the mixture into pure substances. Explain how you would carry out this task. Is your method based on physical or chemical properties? Explain.arrow_forwardWhich of the following represent physical properties or changes, and which represent chemical properties or changes? You curl your hair with a curling iron. You curl your hair by getting a “permanent wave” at the hair salon. Ice on your sidewalk melts when you put salt on it. A glass of water evaporates overnight when it is left on the bedside table. Your steak chars if the skillet is too hot. Alcohol feels cool when it is spilled on the skin. Alcohol ignites when a flame is brought near it. Baking powder causes biscuits to rise.arrow_forwardClassify each of the following as (1) a physical property, (2) a physical change, (3) a chemical property, or (4) a chemical change. a. the process of burning a piece of newspaper b. the fact that metallic copper reacts with chlorine gas c. the process of melting ice d. the fact that metallic gold is a solid at room temperaturearrow_forward
- Decide whether each of the following is a physical property or a chemical property of the substance. a Salt substitute, potassium chloride, dissolves in water. b Seashells, calcium carbonate, fizz when immersed in vinegar. c The gas hydrogen sulfide smells like rotten eggs. d Fine steel wool (Fe) can be burned in air. e Pure water freezes at 0C.arrow_forwardThe following are properties of substances. Decide whether each is a physical property or a chemical property. a Chlorine gas liquefies at 35C under normal pressure. b Hydrogen burns in chlorine gas. c Bromine melts at 7.2C. d Lithium is a soft, silvery-colored metal. e Iron rusts in an atmosphere of moist air.arrow_forwardClassify each of the following as (1) a physical property, (2) a physical change, (3) a chemical property, or (4) a chemical change. a. the process of decomposing hydrogen peroxide b. the fact that a block of ice can be chipped into smaller pieces c. the process of evaporating a liquid d. the fact that water freezes at 32Farrow_forward
- Suppose someone emptied ball bearings into a container of salt. Could you separate the ball bearings from the salt? How? Would your method involve no change, be a physical change, or be a chemical change?arrow_forwardIn each case, identify the italicized property as a physical or chemical property. Give a reason for your choice. The normal color of the element bromine is red-orange. Iron is transformed into rust in the presence of air and water. Dynamite can explode. Aluminum metal, the shiny “foil” you use in the kitchen, melts at 660 °C.arrow_forwardAll of the following processes involve a separation of either a mixture into substances or a compound into elements. For each, decide whether a physical process or a chemical reaction is required. a Sodium metal is obtained from the substance sodium chloride. b Iron filings are separated from sand by using a magnet. c Sugar crystals are separated from a sugar syrup by evaporation of water. d Fine crystals of silver chloride are separated from a suspension of the crystals in water. e Copper is produced when zinc metal is placed in a solution of copper(II) sulfate, a compound.arrow_forward
- Classify each of the following properties as physical or chemical. Explain your reasoning in each case. a. Mercury metal is a liquid at room temperature. b. Sodium metal reacts vigorously with water. c. Water freezes at 0C. d. Gold does not rust. e. Chlorophyll molecules are green in color.arrow_forwardIn each case, decide if the change is a chemical or physical change. (a) A cup of household bleach changes the color of your favorite T-shirt from purple to pink. (b) Water vapor in your exhaled breath condenses in the air on a cold day. (c) Plants use carbon dioxide from the air to make sugar. (d) Butter melts when placed in the Sun.arrow_forwardClassify each of the following properties of the metal magnesium as a physical property or a chemical property. a. Solid at room temperature b. Ignites upon heating in air c. Hydrogen gas is produced when it is dissolved in acids d. Has a density of 1.738 g/cm3 at 20Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY