
CHEMISTRY-TEXT
8th Edition
ISBN: 9780134856230
Author: Robinson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.30CP
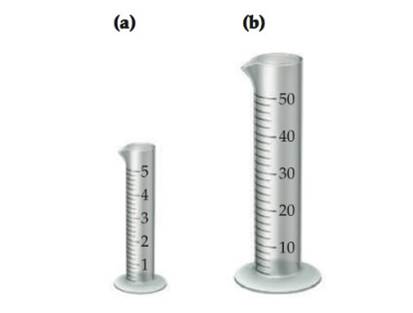
Assume that you have two graduated cylinders, one with a capac- ityof 5 mL (a) and the other with a capacity of 50 mL (b). Draw a line in each, showing how much liquid you would add if you needed to measure 2.64 mL of water. Which cylinder will give the more accurate measurement? Explain.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Highlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area.
HO-
HO-
-0
OH
OH
HO
NG
HO-
HO-
OH
OH
OH
OH
NG
OH
€
+
Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn
it into the product of the reaction.
Also, write the name of the product molecule under the drawing area.
Name: ☐
H
C=0
X
H-
OH
HO-
H
HO-
-H
CH₂OH
×
Draw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than
one anomer, you can draw any of them.
Click and drag to start drawing a
structure.
X
Chapter 1 Solutions
CHEMISTRY-TEXT
Ch. 1 - Express the diameter of a nanoparticle(0.000 000...Ch. 1 - APPLY 1.2 Express the following quantities in...Ch. 1 - PRACTICE 1.3 The melting point of table salt is...Ch. 1 - Prob. 1.4ACh. 1 - PRACTICE 1.5 Chloroform, a substance once used as...Ch. 1 - APPLY 1.6 You are beachcombing on summer vacation...Ch. 1 - PRACTICE 1.7 Some radioactive materials emit a...Ch. 1 - Prob. 1.8ACh. 1 - How many significant figures does each of the...Ch. 1 - Read the volume of the buret and reportyour answer...
Ch. 1 - Examine the figure in Worked Example 1.6. Which...Ch. 1 - A 1.000 mL sample of acetone, a common solvent...Ch. 1 - Carry out the following calculations, expressing...Ch. 1 - APPLY 1.14 A sodium chloride solution was prepared...Ch. 1 - PRACTICE 1.15 Gemstones are weighed in carats,...Ch. 1 - PRACTICE 1.15 Gemstones are weighed in carats,...Ch. 1 - The maximum dimensions of a soccer field are 90.0...Ch. 1 - APPLY 1.18 How large, in cubic centimeters, is the...Ch. 1 - Prob. 1.19PCh. 1 - Prob. 1.20PCh. 1 - Use Figure 1.10 to estimate in powers of 10 (a)...Ch. 1 - On the nanoscale, materials often exhibit...Ch. 1 - Refer to Figure 1.11. Which cube has a...Ch. 1 - Catalytic converters use nanoscale particles of...Ch. 1 - Platinum is an expensive and rare metal used...Ch. 1 - Prob. 1.26PCh. 1 - Which block in each of the following drawings of a...Ch. 1 - Prob. 1.28CPCh. 1 - How many milliliters of water does the graduated...Ch. 1 - Assume that you have two graduated cylinders, one...Ch. 1 - The following cylinder contains three liquids that...Ch. 1 - The following statements pertain to the...Ch. 1 - The following statements pertain to the...Ch. 1 - Label the following statements about the world’s...Ch. 1 - Label the following statements as quantitative or...Ch. 1 - Refer to Figure 1.2. What is developed when...Ch. 1 - What is the difference between a hypothesis and...Ch. 1 - What SI units are used for measuring the following...Ch. 1 - Prefixes for multiples of SI units are used to...Ch. 1 - Prob. 1.40SPCh. 1 - Prob. 1.41SPCh. 1 - Bottles of wine sometimes carry the notation...Ch. 1 - Which quantity in each of the following pairs is...Ch. 1 - Which quantity in each of the following pairs is...Ch. 1 - How many picograms are in 1 mg? In 35 ng?Ch. 1 - How many microliters are in 1 L? In 20 mL?Ch. 1 - Prob. 1.47SPCh. 1 - Express the following measurements in scientific...Ch. 1 - Convert the following measurements from scientific...Ch. 1 - An experimental procedure call for 250 mg of...Ch. 1 - A virus has a diameter of 5.2108m . What is the...Ch. 1 - Which is larger, a Fahrenheit degree or a Celsius...Ch. 1 - What is the difference between a kelvin and a...Ch. 1 - The normal body temperature of a goat is 39.9 °C,...Ch. 1 - Of the 90 or so naturally occurring elements, only...Ch. 1 - Suppose that your oven is calibrated in degrees...Ch. 1 - Tungsten, the element used to make filaments in...Ch. 1 - Suppose you were dissatisfied with both Celsius...Ch. 1 - Answer parts (a)(d) of Problem 1.58 assuming that...Ch. 1 - Sodium chloride has a melting point of 1074 K and...Ch. 1 - A 125 mL sample of water at 293.2 K was heated for...Ch. 1 - What is the difference between a derived SI unit...Ch. 1 - Which volume in each pair is larger, and by...Ch. 1 - What is the volume in L of a cube with an edge...Ch. 1 - What is the volume in mL of a cube with an edge...Ch. 1 - What is the density of glass in g/cm3 if a sample...Ch. 1 - What is the density of lead in g/cm3 if a sample...Ch. 1 - A vessel contains 4.67 L of bromine whose density...Ch. 1 - Aspirin has a density of 1.40g/cm3 . What is the...Ch. 1 - Gaseous hydrogen has a density of 0.0899 g/L at...Ch. 1 - The density of silver is 10.5g/cm3 . What is the...Ch. 1 - Prob. 1.72SPCh. 1 - Prob. 1.73SPCh. 1 - You would like to determine if a set of antique...Ch. 1 - An experiment is performed to determine if pennies...Ch. 1 - The density of chloroform, a widely used organic...Ch. 1 - More sulfuric acid (density=1.8302g/cm3) is...Ch. 1 - Prob. 1.78SPCh. 1 - Assume that the kinetic energy of a 1400 kg car...Ch. 1 - Prob. 1.80SPCh. 1 - Prob. 1.81SPCh. 1 - Prob. 1.82SPCh. 1 - Prob. 1.83SPCh. 1 - Prob. 1.84SPCh. 1 - What is the difference in mass between a nickel...Ch. 1 - How many significant figures are in each of the...Ch. 1 - Prob. 1.87SPCh. 1 - Prob. 1.88SPCh. 1 - The diameter of the Earth at the equator is...Ch. 1 - Round off the following quantities to the number...Ch. 1 - Round off the following quantities to the number...Ch. 1 - Express the results of the following calculations...Ch. 1 - Express the results of the following calculations...Ch. 1 - Carry out the following conversions. (a) How many...Ch. 1 - Convert the following quantities into SI units...Ch. 1 - Prob. 1.96SPCh. 1 - In the United States, the emissions limit for...Ch. 1 - Prob. 1.98SPCh. 1 - Prob. 1.99SPCh. 1 - Prob. 1.100SPCh. 1 - Concentrations of substances dissolved in solution...Ch. 1 - Prob. 1.102SPCh. 1 - Prob. 1.103SPCh. 1 - Which is larger in each pair, and by approximate...Ch. 1 - The density of polystyrene, a plastic commonly...Ch. 1 - The density of polypropylene, a plastic commonly...Ch. 1 - Prob. 1.107MPCh. 1 - A 1.0-ounce piece of chocolate contains 15 mg of...Ch. 1 - Prob. 1.109MPCh. 1 - Prob. 1.110MPCh. 1 - Prob. 1.111MPCh. 1 - A bag of Hershey’s Kisses contains the following...Ch. 1 - Vinaigrette salad dressing consists mainly of oil...Ch. 1 - At a certain point, the Celsius and Fahrenheit...Ch. 1 - Prob. 1.115MPCh. 1 - A calibrated flask was filled to the 25.00 mL mark...Ch. 1 - Brass is a copper-zinc alloy. What is the mass in...Ch. 1 - Ocean currents are measured in Sverdrups (sv)...Ch. 1 - The element gallium (Ga) has the second-largest...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Epoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forwardYou may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward
- 1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forwardCalculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forwardAlcohols can be synthesized using an acid-catalyzed hydration of an alkene. An alkene is combined with aqueous acid (e.. sulfuric acid in water). The reaction mechanism typically involves a carbocation intermediate. > 3rd attempt 3343 10 8 Draw arrows to show the reaction between the alkene and hydronium ion. that 2nd attempt Feedback 1st attempt تعمال Ju See Periodic Table See Hint F D Ju See Periodic Table See Hintarrow_forward
- Draw the simplified curved arrow mechanism for the reaction of acetone and CHgLi to give the major product. 4th attempt Π Draw the simplified curved arrow mechanism T 3rd attempt Feedback Ju See Periodic Table See Hint H -H H -I H F See Periodic Table See Hintarrow_forwardSelect the correct reagent to accomplish the first step of this reaction. Then draw a mechanism on the Grignard reagent using curved arrow notation to show how it is converted to the final product. 4th attempt Part 1 (0.5 point) Select the correct reagent to accomplish the first step of this reaction. Choose one: OA Mg in ethanol (EtOH) OB. 2 Li in THF O C. Li in THF D. Mg in THF O E Mg in H2O Part 2 (0.5 point) Br Part 1 Bri Mg CH B CH, 1 Draw intermediate here, but no arrows. © TE See Periodic Table See Hint See Hint ין Harrow_forwardSelect the product for the following reaction. HO HO PCC OH ○ OH O HO ○ HO HO HOarrow_forward
- 5:45 Х Select the final product for the following reaction sequence. O O 1. Mg. ether 2.D.Oarrow_forwardBased on the chart Two similarities between the molecule with alpha glycosidic linkages. Two similarities between the molecules with beta glycosidtic linkages. Two differences between the alpha and beta glycosidic linkages.arrow_forwardplease help fill in the tablearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Measurement and Significant Figures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=Gn97hpEkTiM;License: Standard YouTube License, CC-BY
Trigonometry: Radians & Degrees (Section 3.2); Author: Math TV with Professor V;https://www.youtube.com/watch?v=U5a9e1J_V1Y;License: Standard YouTube License, CC-BY