
EBK CHEMISTRY: THE MOLECULAR NATURE OF
7th Edition
ISBN: 9781119513216
Author: HYSLOP
Publisher: JOHN WILEY+SONS INC.
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 10RQ
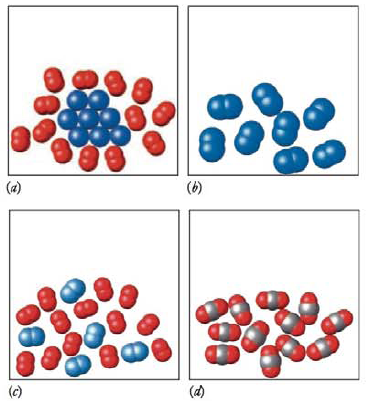
For each of the following molecular pictures, state whether it represents a pure substance or a mixture. For pure substances, state whether it represents an element or a compound. For mixtures, state whether it is homogeneous or heterogeneous.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Question 6 of 7 (1 point) | Question Attempt: 1 of 1
= 1
✓2
✓ 3
✓ 4
✓ 5
6
✓ 7
This organic molecule is dissolved in a basic aqueous solution:
Jen ✓
?
A short time later sensitive infrared spectroscopy reveals the presence of a new C-OH stretch absorption. That is,
must now be a new molecule present with at least one C- OH bond.
there
18
In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule Ar
©
+
Click and drag to start
drawing a structure.
Add/Remove step
Click and
drawing
Save For Later
Submit Assignment
Can you please explain this problem to me? I'm very confused about it. Please provide a detailed, step-by-step explanation for me! (ME EX1) Prblm 22
Can you please explain this problems to me? I'm very confused about it. Please provide a detailed, step-by-step explanation for me! (ME EX1) Prblm 30
Chapter 1 Solutions
EBK CHEMISTRY: THE MOLECULAR NATURE OF
Ch. 1 - Practice Exercise 1.1
What is the process of the...Ch. 1 - Practice Exercise 1.2
Classify the following as an...Ch. 1 - Practice Exercise 1.3
Each of the following can be...Ch. 1 - Practice Exercise 1.4
Each of these properties can...Ch. 1 - Prob. 5PECh. 1 - Prob. 6PECh. 1 - What is 355CinF? (Hint: What tool relates these...Ch. 1 - Convert 55F to its Celsius temperature. What...Ch. 1 - Prob. 9PECh. 1 - Perform the following calculations involving...
Ch. 1 - Practice Exercise 1.11
Use dimensional analysis to...Ch. 1 - Use dimensional analysis to perform the following...Ch. 1 - A 15.0 mL sample of polystyrene used in insulated...Ch. 1 - Practice Exercise 1.14
A crystal of salt was grown...Ch. 1 - A gold-colored metal object has a mass of 365 g...Ch. 1 - Practice Exercise 1.16
A certain metal alloy has a...Ch. 1 - Practice Exercise 1.17
Table wines have a minimum...Ch. 1 - Practice Exercise 1.18
Diabetes can cause a...Ch. 1 - After some thought, give two reasons why a course...Ch. 1 - What steps are involved in the scientific method?Ch. 1 - Prob. 3RQCh. 1 - Can a theory be proved to be correct? Can a theory...Ch. 1 - 1.5 Define matter. Which are examples of matter?...Ch. 1 - Define (a) element, (b) compound, (c) mixture, (d)...Ch. 1 - 1.7 Which kind of change, chemical or physical, is...Ch. 1 - Prob. 8RQCh. 1 - 1.9 What is the name of each of the following...Ch. 1 - For each of the following molecular pictures,...Ch. 1 - 1.11 Consider the following four samples of...Ch. 1 - 1.12 What is a physical change? What is a chemical...Ch. 1 - 1.13 “A sample of calcium (an electrically...Ch. 1 - 1.14 In places like Saudi Arabia, freshwater is...Ch. 1 - How does a chemical property differ from a...Ch. 1 - Distinguish between an extensive and an intensive...Ch. 1 - Determine whether each of the following is an...Ch. 1 - Describe one or more physical properties of each...Ch. 1 - Why must measurements always be written with a...Ch. 1 - What is the only SI base unit that includes a...Ch. 1 - Which SI units are mainly used in chemistry?Ch. 1 - What are derived units? Give two examples of...Ch. 1 - What is the meaning of each of the following...Ch. 1 - What reference points do we use in calibrating the...Ch. 1 - In each pair, which is larger: (a) A Fahrenheit...Ch. 1 - 1.26 Define the term significant figures.
Ch. 1 - Explain how to round numbers.Ch. 1 - What is the difference between accuracy and...Ch. 1 - 1.29 Suppose a length had been reported to be...Ch. 1 - What is the difference between the treatment of...Ch. 1 - When constructing a conversion factor between two...Ch. 1 - Suppose someone suggested using the fraction 3...Ch. 1 - In 1 hour there are 3600 seconds. By what...Ch. 1 - If you were to convert the measured length 4.165...Ch. 1 - 1.35 Write the equation that defines density....Ch. 1 - Compare density and specific gravity. What is the...Ch. 1 - 1.37 Give four sets of units for density. What...Ch. 1 - 1.38 Silver has a density of . Express this as an...Ch. 1 - Determine whether each of the following is a...Ch. 1 - 1.40 Determine whether each of the following is a...Ch. 1 - At room temperature, what is the state of each of...Ch. 1 - At room temperature, determine the appropriate...Ch. 1 - What number should replace the question mark in...Ch. 1 - 1.44 What numbers should replace the question...Ch. 1 - Perform the following conversions. (a) 57CtoF (b)...Ch. 1 - Perform the following conversions. (a) 98FtoC (b)...Ch. 1 - The temperature of the core of the sun is...Ch. 1 - Natural gas is mostly methane, a substance that...Ch. 1 - A healthy dog has a temperature ranging from...Ch. 1 - Prob. 50RQCh. 1 - The length of a wire was measured using two...Ch. 1 - What are the temperatures being measured in the...Ch. 1 - 1.53 How many significant figures do the following...Ch. 1 - How many significant figures do the following...Ch. 1 - 1.55 Perform the following arithmetic and round...Ch. 1 - 1.56 Perform the following arithmetic and round...Ch. 1 - Which are exact numbers and which ones have a...Ch. 1 - Prob. 58RQCh. 1 - 1.59 Perform the following conversions.
Ch. 1 - 1.60 Perform the following conversions.
Ch. 1 - 1.61 Perform the following conversions. If...Ch. 1 - 1.62 Perform the following conversions. If...Ch. 1 - 1.63 Perform the following conversions....Ch. 1 - Prob. 64RQCh. 1 - The human stomach can expand to hold up to 4.2...Ch. 1 - In the movie Cool Hand Luke (1967), Luke wagers...Ch. 1 - 1.67 The winds in a hurricane can reach almost 200...Ch. 1 - 1.68 A bullet is fired at a speed of 2435 ft/s....Ch. 1 - 1.69 A bullet leaving the muzzle of a pistol was...Ch. 1 - 1.70 On average, water flows over Niagara Falls at...Ch. 1 - Prob. 71RQCh. 1 - *1.72 One degree of latitude on the earth’s...Ch. 1 - Calculate the density of kerosene, in g/mL, if its...Ch. 1 - Calculate the density of magnesium, in g/cm3, if...Ch. 1 - Acetone, the solvent in some nail polish removers,...Ch. 1 - A glass apparatus contains 26.223 g of water when...Ch. 1 - 1.77 Chloroform, a chemical once used as an...Ch. 1 - Gasolines density is about 0.65 g/mL. How much...Ch. 1 - Prob. 79RQCh. 1 - Prob. 80RQCh. 1 - The space shuttle uses liquid hydrogen as its...Ch. 1 - Prob. 82RQCh. 1 - 1.83 Some time ago, a U.S. citizen traveling in...Ch. 1 - Driving to work one day, one of the authors of...Ch. 1 - Prob. 85RQCh. 1 - Prob. 86RQCh. 1 - Prob. 87RQCh. 1 - A pycnometer is a glass apparatus used for...Ch. 1 - 1.89 Radio waves travel at the speed of light, ....Ch. 1 - Prob. 90RQCh. 1 - Prob. 91RQCh. 1 - Aerogel or solid smoke" is a novel material that...Ch. 1 - A liquid known to be either ethanol (ethyl...Ch. 1 - An unknown liquid was found to have a density of...Ch. 1 - 1.95 There exists a single temperature at which...Ch. 1 - In the text, the Kelvin scale of temperature is...Ch. 1 - Density measurements can be used to analyze...Ch. 1 - An artist's statue has a surface area of 14.6ft2....Ch. 1 - What is the volume in cubic millimeters of a 3.54...Ch. 1 - 1.100 A solution is defined as a uniform mixture...Ch. 1 - How do you know that Coca-Cola is not a compound?...Ch. 1 - Find two or more web sites that give the values...Ch. 1 - 1.103 Reference books such as the Handbook of...Ch. 1 - A student used a 250 mL graduated cylinder having...Ch. 1 - Prob. 105RQCh. 1 - Gold has a density of 19.31gcm-3. How many grams...Ch. 1 - A Boeing 747 jet airliner carrying 568 people...Ch. 1 - *1.108 Download a table of data for the density of...Ch. 1 - List the physical and chemical properties...Ch. 1 - Prob. 110RQ
Additional Science Textbook Solutions
Find more solutions based on key concepts
91. Classify each molecule as polar nonpolar.
a.
b.
c.
d.
Introductory Chemistry (6th Edition)
1. What is thermochemistry? Why is it important?
Chemistry: Structure and Properties (2nd Edition)
Practice Problem 2.15 Eugenol is the main constituent of the natural oil from cloves. Circle and label all of t...
Organic Chemistry
Your bore cells, muscle cells, and skin cells look different because a. different kinds of genes are present in...
Campbell Essential Biology (7th Edition)
The following data were obtained from a disk-diffusion test. Antibiotic Zone of Inhibition A 15 mm B 0 mm c 7 m...
Microbiology: An Introduction
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- This organic molecule is dissolved in a basic aqueous solution: O ? olo RET A short time later sensitive infrared spectroscopy reveals the presence of a new C-OH stretch absorption. That is, there Ar must now be a new molecule present with at least one C - OH bond. In the drawing area below, show the detailed mechanism that could convert the molecule above into the new molecule. $ Add/Remove steparrow_forwardSo the thing is im trying to memorize VESPR Shapes in order to be able to solve problems like so, and I need help with making circles like the second image that's in blue or using an x and y axis plane in order to memorize these and be able to solve those type of problems. Especially like the ones given in the top / first image. (180 , 120 , 109.5) Can you help me with this.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- 2. (15 points) Draw an appropriate mechanism for the following reaction. H N. H* + H₂Oarrow_forwardDraw a tripeptide of your choosing at pH 7. Have the N-terminus on the left and the C-terminus on the right. Then: Draw a triangle around the α-carbons. Draw a box around the R-groups. Circle the atoms capable of hydrogen bonding. Highlight the atoms involved in the formation of the peptide bonds. What type of structure have you drawn? (primary, secondary, tertiary or quaternary protein structure). make sure its a tripeptidearrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward
- Don't used hand raiting and don't used Ai solutionarrow_forwardDon't used Ai solution and don't used hand raitingarrow_forward> Organic Functional Groups Naming and drawing alkyl halides structure CI Br CI CI Explanation Check 2 name 1-chloro-2,4,9-trimethylnonane CI 2-iodo-2,3-dimethylbutane FEB 19 € E M tv MacBook Airarrow_forward
- Can you please explain to me this problem im very confused and lost. Help me step by step and in detail im soo lost.arrow_forward2) There are many forms of cancer, all of which involve abnormal cell growth. The growth and production of cells, called cell proliferation, is known to involve an enzyme called protein farnesyltransferase (PFTase). It is thought that inhibitors pf PFTase may be useful as anticancer drugs. The following molecule showed moderate activity as a potential PFTase inhibitor. Draw all stereoisomers of this compound. HO OHarrow_forwardConsidering rotation around the bond highlighted in red, draw the Newman projection for the most stable and least stable conformations when viewed down the red bond in the direction of the arrow. Part 1 of 2 H₁₂C H H Draw the Newman projection for the most stable conformation. Select a template to begin. Part 2 of 2 Draw the Newman projection for the least stable conformation. G 心arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY