Draw the product of the following reaction:

When the carbon-carbon double bond has an amine substituent, it is termed as enamine. The mild acid-catalyzed reaction of the carbonyl compound that has alpha hydrogen to a secondary amine furnishes the enamine. The resonance structure of the enamine produces the carbanion and hence enamine functions as a good nucleophile. Besides, the enamine can also behave as a base in the abstraction of protons. The notable application of enamine formation of the carbonyl compound is to functionalize the alpha carbon by nucleophilic addition or substitution reaction.
Reaction:
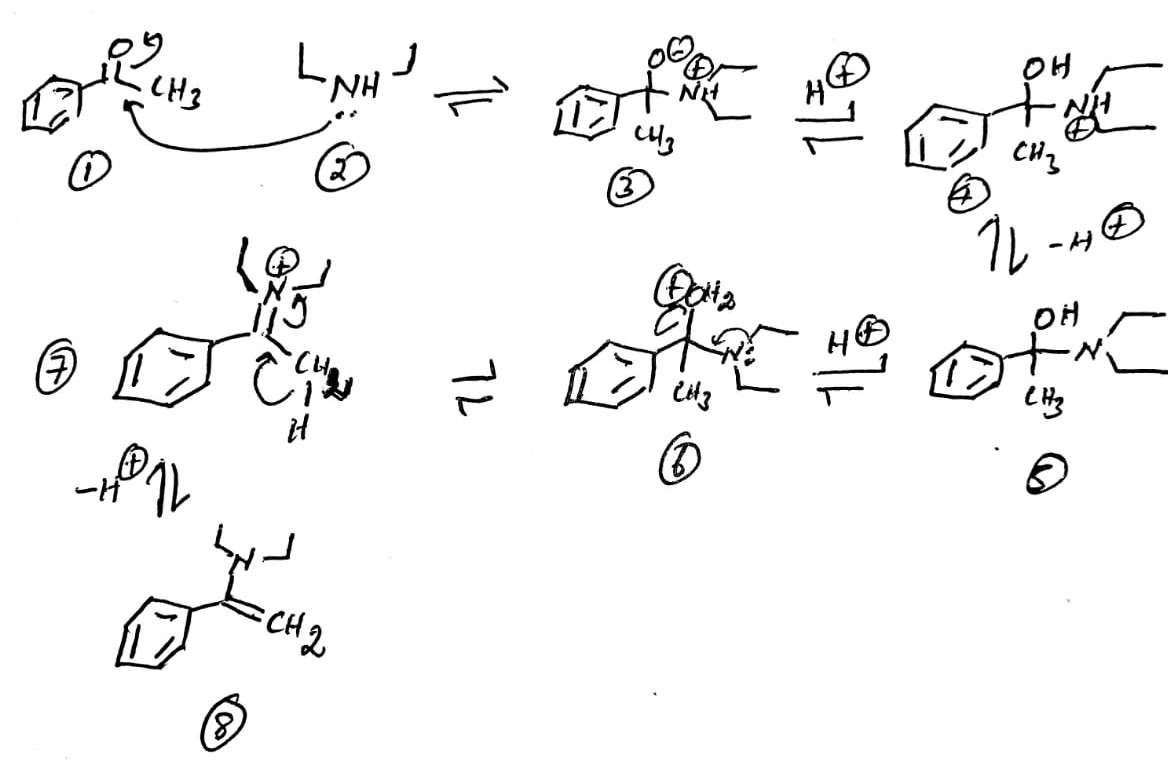
Explanation:
The nucleophilic addition of secondary amine 2 to acetophenone 1 leads to the formation of ammonium ion 3. The proton exchange produces the free -OH unit 4. The removal of the proton from 4 yields the free amine 5. The protonation of -OH by the acid produces 6. The shift of lone pair from nitrogen towards the carbon removes the water molecule to provide 7. The removal of alpha hydrogen as a hydrogen ion () and the migration of the bonded electrons towards the carbon-nitrogen bond produces the enamine 8.
Step by step
Solved in 3 steps with 1 images









