The rate of the reaction A + 3B → C + 2D was reported as 2.7 M/sec. Calculate the rates of formation and consumption of each of the reactants and products. (include negative signs where appropriate). 2. The rate of the following disproportionation redox reaction was studied by monitoring the rate of formation of MnO4- 3 MnO42- (aq) + H+(aq) --> 2 MnO4-(aq) + MnO2(s) At the 5.30 minute mark of the reaction the concentration of MnO4- was found to be 0.52 M. At the 6.25 minute mark of the reaction the concentration of MnO4- was found to be 1.75 M. a. What is the rate of appearance of MnO4-? b. What is the rate of disappearance of MnO42-? c. the rate of formation of MnO2 is how many times the rate of formation of MnO4-? d. What is the value of the rate of reaction?
Please answer all parts!
Background:
1. The rate of the reaction A + 3B → C + 2D was reported as 2.7 M/sec. Calculate the rates of formation and consumption of each of the reactants and products. (include negative signs where appropriate).
2. The rate of the following disproportionation
3 MnO42- (aq) + H+(aq) --> 2 MnO4-(aq) + MnO2(s)
At the 5.30 minute mark of the reaction the concentration of MnO4- was found to be 0.52 M. At the 6.25 minute mark of the reaction the concentration of MnO4- was found to be 1.75 M.
a. What is the rate of appearance of MnO4-?
b. What is the rate of disappearance of MnO42-?
c. the rate of formation of MnO2 is how many times the rate of formation of MnO4-?
d. What is the value of the
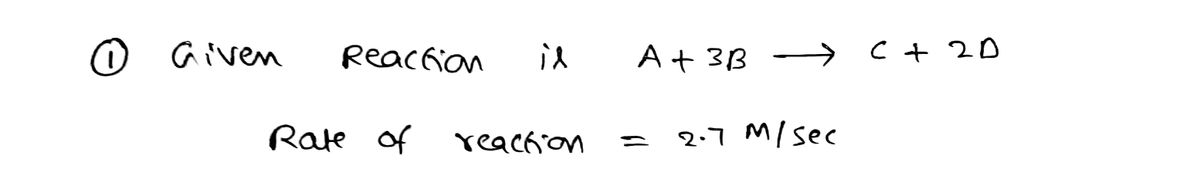
Step by step
Solved in 6 steps with 6 images









