Suppose a heat engine is connected to two energy reservoirs, one a pool of molten aluminum (660°C) and the other a block of solid mercury (−38.9°C). The engine runs by freezing 1.40 g of aluminum and melting 16.0 g of mercury during each cycle. The heat of fusion of aluminum is 3.97 105 J/kg; the heat of fusion of mercury is 1.18 104 J/kg. What is the efficiency of this engine? %
Q: A certain coal-fired power plant has a rated power capacity of P = 950 MW. The output of this plant…
A:
Q: An automobile engine converts heat into work via a cycle. The cycle must finish exactly where it…
A:
Q: You observe a grasshopper eating a blade of grass. You know that each blade of grass contains 100…
A: Given that:- Energy contain in one blade of grass=100 units
Q: A 2000kg car is advertised that it can go from rest to 30m/s, drives 100km, and 3000m in height on 1…
A: Part (a): The 1 gram of gasoline gives 5×104 J of heat energy. Hence, the total heat energy (Q)…
Q: A heat engine using 1.0 mol of a monatomic gas follows the cycle shown. 3750 J of heat energy is…
A:
Q: The specific heat of water is 4186 J/kg c°. How much does the internal energy of 200 g of water…
A:
Q: This problem compares the energy output and heat transfer to the environment by two different types…
A: The efficiency of the first nuclear power plant is, The efficiency of the second nuclear power plant…
Q: After you drive for a while your tires will gain some energy via friction and heat up. If the air…
A: Given Heat added Q=150J Work done by gas W=100J
Q: Suppose a heat engine is connected to two energy reservoirs, one a pool of molten aluminum (660°C)…
A: Given data The temperature of the molten aluminum is Tm_Al = 660°C The temperature of the solid…
Q: The ice maker inside a refrigerator makes ice cubes at 0.0°C from water that is at 17.2°C when it…
A: Given data The temperature of the water is Tw=17.2°C. The temperature of the ice is Ti=0°C. The…
Q: The work from a Carnot engine between two reservoirs at TH=847 K and T=523 K is used to operate a…
A: Solution: Given Values, TH=847 KTL=523 K
Q: One mole of an ideal gas first undergoes an isothermal expansion at a temperature T1 = 650 K to 5…
A:
Q: A sealed container containing water with stirring wings inside is heating up on the stove. In this…
A:
Q: A gasoline engine takes in 1.61×104 J of heat and delivers 3700 J of work per cycle. The heat is…
A:
Q: expands, it does 280 J of work. What is the change in the internal energy AU of the gas during this…
A: For an ideal gas, internal energy is only the function of temperature. And given that we have…
Q: 3 A mixture of fuel and air is enclosed in an engine cylinder fitted with a piston. The gas pressure…
A:
Q: The interior of a refrigerator has a surface area of 5.6 m2 . It is insulated by a 2.3 cm thick…
A: The rate Hcond at which energy is conducted through a material whose faces are maintained at…
Q: If the plant operates at 50.0% of its maximum efficiency and its power output is 1.23 × 108 W, at…
A: The given data are: TH=540 KTL=313 KQH=1.23×108 W Here, TH is the higher temperature, TL is the…
Q: Converting sunlight to electricity with solar cells has an efficiency of 15%. It's possible to…
A:
Q: A power plant taps steam superheated by geothermal energy to 558 K (the temperature of the hot…
A: Given data: Temperature of hot reservoir, Th=558 K Temperature of cold reservoir, Tc=305 K Output…
Q: The hot reservoir for a Carnot engine has a temperature of 943 k, while the cold reservoir has a…
A: Solution Given dataFor first heat engine Temperature of hot reservoir TH1=943KTemperature of cold…
Q: A 53-kg mountain climber, starting from rest, climbs a vertical distance of 764 m. At the top, she…
A:
Q: A lab rat lifts a 200 g mass 1.55 m high as it spins on a Ferris wheel. At the same time, it loses…
A:
Q: 3.5x10^7 J of heat transfers into an engine. If 2.1x10^7 J of work is performed what is the…
A:
Q: A heat engine operates under the following conditions: heat exchanged via exhaust Qc = -2500 J, work…
A: Given:- heat energy = Qc = -2500 J W = -1000 J
Q: An electrical power plant is constructed to extract energy from the ocean. It utilizes warm water at…
A: Solution: Given: t1 = temperature of warm water = 30oC t2 = temperature of cold water = 4.1oC η1 =…
Q: The hot reservoir for a Carnot engine has a temperature of 904 K, while the cold reservoir has a…
A: Given,temperature of hot reservoir for Engine 1, ,Temperature of cold reservoir for Engine 1, ,Heat…
Q: 0.90 L of 25 °C water is placed in a refrigerator. The refrigerator's motor must supply an extra 9.0…
A: Approach to solving the question:Please see attached photos for detailed solutions. thank you.…
Q: One pound of air per second with an initial temperature of 180°F is allowed to expand without flow…
A:
Q: An engine has a hot reservoir that contains 20,000 J of heat energy at 3270 C, and a cold reservoir…
A:
Q: A freezer has a coefficient of performance of 5.7. You place 0.45 kg of water at 21°C in the…
A:
Q: The ice maker inside a refrigerator makes ice cubes at 0.0°C from water that is at 14.2°C when it…
A: Given, Temperature of refrigerator,T2=0°C Temperature of water,T1=14.2 °C Power of machine,P=184 W…
Q: A heat pump is a heating system installed on some newer houses. It works by pumping heat from…
A: Given that: ηHP max=THTH-TCTo=44°F=279.82 KTin=65°F=291.48 KA=1025 ft2h=8 ft
Q: Problem 1: Which of the following would violate the Second Law of Thermodynamics? Choose one that…
A: The second law of thermodynamics states that any spontaneously occurring process will always lead to…
Suppose a heat engine is connected to two energy reservoirs, one a pool of molten aluminum (660°C) and the other a block of solid mercury (−38.9°C). The engine runs by freezing 1.40 g of aluminum and melting 16.0 g of mercury during each cycle. The heat of fusion of aluminum is 3.97 105 J/kg; the heat of fusion of mercury is 1.18 104 J/kg. What is the efficiency of this engine?
%
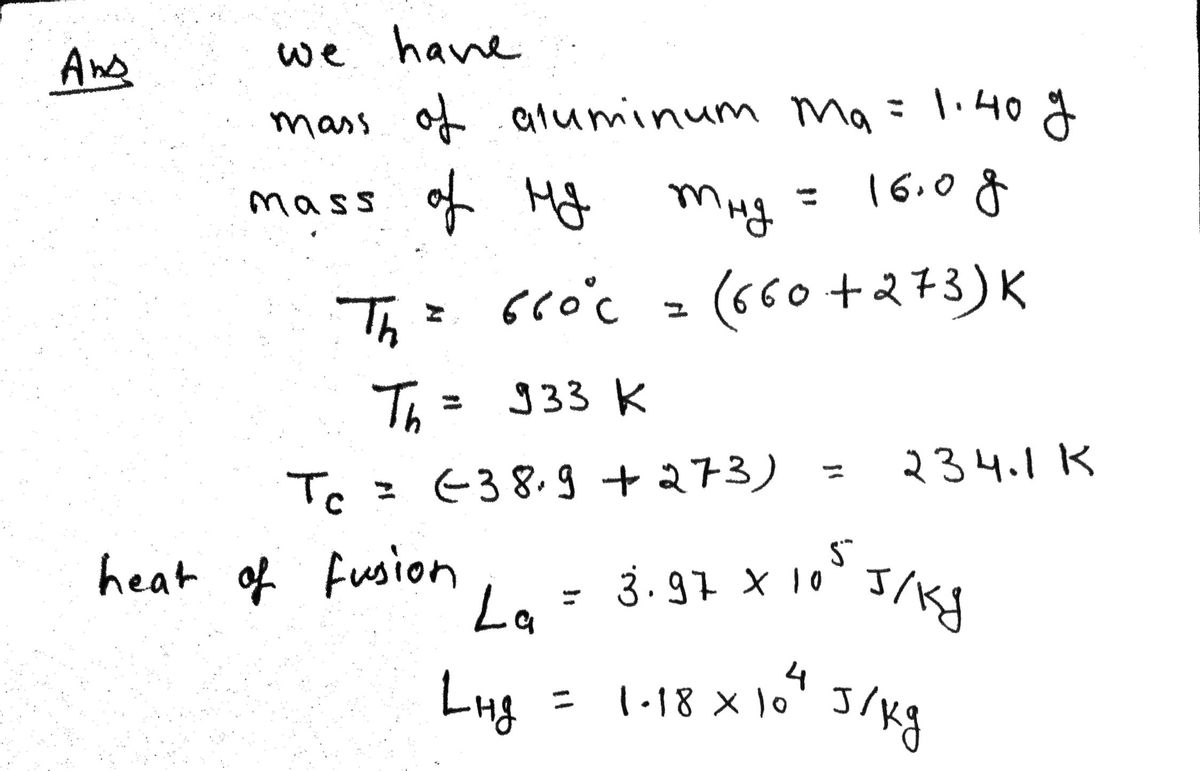
Step by step
Solved in 3 steps with 3 images

- In performing 154.0 J of work, an engine exhausts 65.0 J of heat. What is the efficiency of the engine? e = 29A typical coal-fired power plant burns 340 metric tons of coal every hour to generate 2.5 × 106 MJ of energy. One metric ton has a mass of 1000 kg and a metric ton of coal has a volume of 1.5 m^3. The heat of combustion is 28 MJ/kg. What is the power plant’s efficiency?Two engines operate between the same two temperatures of 787 K and 385 K, and have the same rate of heat input. One of the engines is a reversible engine with a power output of 23.0 kW. The second engine has an efficiency of 42.0%. What is the power output of the second engine?
- A system with a piston expands when it gives off 51.9J of heat to the surroundings. The piston is working against a pressure of .581 atm. The final volume is 58.6L and the initial volume was 65.1L. What is the change in internal energy?An electric motor has its shaft coupled to that of an electric generator. The motor drives the generator, and some current from the generator is used to run the motor. The excess current is used to light a home. What is wrong with this scheme?Suppose that you have left a 200 mL cup of coffee sitting until it has cooled to 30◦ C, which you find totally unacceptable. Your microwave oven draws 1100W of electrical power when it is running. If it takes 45s for this microwave oven to raise the temperature of the coffee to 60◦ C, what is the efficiency of heating with this oven? You will need to use the fact that 4.2 J of energy is required to raise the temperature of 1.0 mL of coffee by 1.0◦ C.
- An aircraft engine takes in 8900 JJ of heat and discards 6700 JJ each cycle. Part A. What is the mechanical work output of the engine during one cycle? Part B. What is the thermal efficiency of the engine? Express your answer as a percentage.4. With 2.56 × 10^6 J of heat transfer into an engine, it performs only 1.50 × 10^5 J of work. (a)What is the engine’s efficiency? (b) How much heat transfer to the environment takes place?If mass of air in a house is 278.6376kg. And the specific heat of air is 1.0kj/kgK. How much heat energy is needed to heat air by 10K? Use Q=mc∆T
- The hot reservoir for a Carnot engine has a temperature of 938 K, while the cold reservoir has a temperature of 362 K. The heat input for this engine is 7110 J. The 362-K reservoir also serves as the hot reservoir for a second Carnot engine. This second engine uses the rejected heat of the first engine as input and extracts additional work from it. The rejected heat from the second engine goes into a reservoir that has a temperature of 213 K. Find the total work delivered by the two engines.1. A gasoline engine takes in 1.61 x 104 J of heat and delivers 3700 J of work per cycle. The heat is obtained by burning gasoline with a heat of combustion of 4.6 x 104 J/g. (a) What is the thermal efficiency? (b) How much heat is discarded in each cycle? (c) What mass of fuel is burned in each cycle? (d) If the engine goes through 60.0 cycles per second, what is its power output in kilowatts? in horsepower?Copy of A heat engine is used to convert heat into work. If it draws heat from a bath at 500 K, converts it to work, then delivers the remaining heat into a bath at 300 K, what is its maximum possible efficiency? 20% 30% 40% 60% 70% 80% O O O O O