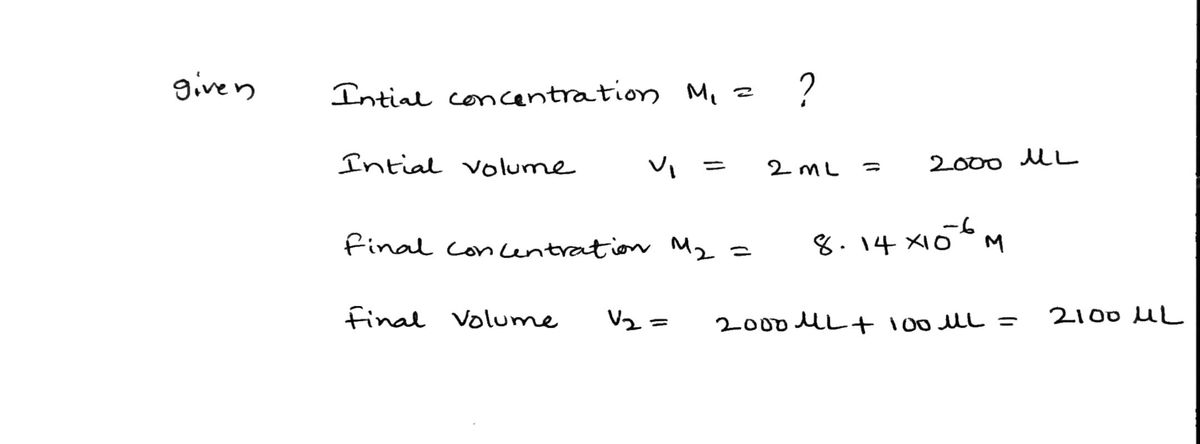
Submit Part C Dilution as a procedure in analysis We sometimes purposely dilute solutions if the analyte concentrations are too high to permit an accurate measurement. For example, a spectrophotometer can measure the amount of light that passes through a sample solution for wavelengths in the visible (and sometimes ultraviolet) range. Based on the dissolved solute's properties, we can quantify that solute's concentration. If the solute's concentration is too high, a relatively small amount of incident light having the wavelength that corresponds to the solute's absorption behavior will reach the detector, which results in an inaccurate measurement (consider the result if you blocked the light with an opaque disc). If the solution is diluted, less light is absorbed, and the detector in the spectrophotometer can generate more reliable data. When talking about small quantities, it is important to understand the relationship between common prefixes. Here are some relationships that may help when working through Part C. 1L = 1,000,000 μL 1000 μL openvellum.ecollege.com HA original concentration of Fe²+ = Value Submit 1000 mL OR 0.001 L 1 mL OR 1x 10 L= 0.001 mL The absorbance of a cationic iron(II) sample solution was measured in a spectrophotometer, but the instrument returned an error because the absorbance was too high. The sample was then diluted by using a pipette to take 100.0 μL of the sample and injecting it into a cuvette already containing 2.00 mL of water (total volume is 2.00 mL + 100.0 μL). The absorbance value of the diluted solution corresponded to a concentration of 8.14x10-6 M. What was the concentration of the original solution? Express the concentration to three significant figures with the appropriate units. ▸ View Available Hint(s) Units = ? 1 μL Ⓒ a P Pearson Copyright © 2022 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions | Contact Us |
Submit Part C Dilution as a procedure in analysis We sometimes purposely dilute solutions if the analyte concentrations are too high to permit an accurate measurement. For example, a spectrophotometer can measure the amount of light that passes through a sample solution for wavelengths in the visible (and sometimes ultraviolet) range. Based on the dissolved solute's properties, we can quantify that solute's concentration. If the solute's concentration is too high, a relatively small amount of incident light having the wavelength that corresponds to the solute's absorption behavior will reach the detector, which results in an inaccurate measurement (consider the result if you blocked the light with an opaque disc). If the solution is diluted, less light is absorbed, and the detector in the spectrophotometer can generate more reliable data. When talking about small quantities, it is important to understand the relationship between common prefixes. Here are some relationships that may help when working through Part C. 1L = 1,000,000 μL 1000 μL openvellum.ecollege.com HA original concentration of Fe²+ = Value Submit 1000 mL OR 0.001 L 1 mL OR 1x 10 L= 0.001 mL The absorbance of a cationic iron(II) sample solution was measured in a spectrophotometer, but the instrument returned an error because the absorbance was too high. The sample was then diluted by using a pipette to take 100.0 μL of the sample and injecting it into a cuvette already containing 2.00 mL of water (total volume is 2.00 mL + 100.0 μL). The absorbance value of the diluted solution corresponded to a concentration of 8.14x10-6 M. What was the concentration of the original solution? Express the concentration to three significant figures with the appropriate units. ▸ View Available Hint(s) Units = ? 1 μL Ⓒ a P Pearson Copyright © 2022 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions | Contact Us |
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:3
Submit
E
Dilution as a procedure in analysis
We sometimes purposely dilute solutions if the analyte concentrations are too high to permit an accurate measurement. For example, a spectrophotometer can measure the amount of
light that passes through a sample solution for wavelengths in the visible (and sometimes ultraviolet) range. Based on the dissolved solute's properties, we can quantify that solute's
concentration. If the solute's concentration is too high, a relatively small amount of incident light having the wavelength that corresponds to the solute's absorption behavior will reach the
detector, which results in an inaccurate measurement (consider the result if you blocked the light with an opaque disc). If the solution is diluted, less light is absorbed, and the detector in
the spectrophotometer can generate more reliable data.
When talking about small quantities, it is important to understand the relationship between common prefixes. Here are some relationships that may help when working through Part C.
1L = 1000 mL
1000 mL = 1,000,000 μL
OR
Part C
original concentration of Fe²+ = Value
Submit
80
F3
$
The absorbance of a cationic iron(II) sample solution was measured in a spectrophotometer, but the instrument returned an error because the absorbance was too high. The sample
was then diluted by using a pipette to take 100.0 μL of the sample and injecting it into a cuvette already containing 2.00 mL of water (total volume is 2.00 mL + 100.0 μL). The
absorbance value of the diluted solution corresponded to a concentration of 8.14x10-6 M. What was the concentration of the original solution?
Express the concentration to three significant figures with the appropriate units.
View Available Hint(s)
4
R
F4
D F
%
0⁰4 μÅ 6
5
F5
openvellum.ecollege.com
T
G
^
0.001 L = 1 mL
OR
1x 106 L = 0.001 mL
Copyright © 2022 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions | Contact Us |
6
F6
C www. ?
Units
MacBook Air
Y
H
&
7
P Pearson
F7
U
=
*
=
8
J
1000 μL
FB
1 μL
1
(
9
K
DD
FO
O
)
0
L
J
F10
1
P
(1
F11
+
F12
11
+
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY