Salt can be made from seawater by solar evaporation. In the first stage of the process, all of the CaCO3 and CaSO4 are precipitated and then the solution is sent to a crystallization pond. In the crystallization pond, water is evaporated and crystalline NaCl is recovered. The pond outlets are a stream containing 91% of the inlet NaCl (pure NaCl crystals) and a stream of mother liquor (the remaining solution), which has an sg of 1.26. The total salt content of the inlet sea water is 3.7 wt% with an sg of 1.03. The composition of the inlet sea water on a dry basis is 78 wt% NaCl, 11 wt% MgCl2, 4.5 wt% MgSO4, 3.5 wt% CaSO4, 2.5 wt% K2SO4, 0.3 wt% CaCO3 and 0.2 wt% MgBr2. Assume that the losses of NaCl, MgSO4, MgCl2, MgBr2, and K2SO4 are negligible. a) Calculate the water (ton/hr) evaporated for an inlet sea water flowrate of 1 ton/hr. b) Calculate the concentration (wt% total salt) of the mother liquor.
Salt can be made from seawater by solar evaporation. In the first stage of the process, all of the CaCO3 and CaSO4 are precipitated and then the solution is sent to a crystallization pond. In the crystallization pond, water is evaporated and crystalline NaCl is recovered. The pond outlets are a stream containing 91% of the inlet NaCl (pure NaCl crystals) and a stream of mother liquor (the remaining solution), which has an sg of 1.26.
The total salt content of the inlet sea water is 3.7 wt% with an sg of 1.03. The composition of the inlet sea water on a dry basis is 78 wt% NaCl, 11 wt% MgCl2, 4.5 wt% MgSO4, 3.5 wt% CaSO4, 2.5 wt% K2SO4, 0.3 wt% CaCO3 and 0.2 wt% MgBr2. Assume that the losses of NaCl, MgSO4, MgCl2, MgBr2, and K2SO4 are negligible. a) Calculate the water (ton/hr) evaporated for an inlet sea water flowrate of 1 ton/hr. b) Calculate the concentration (wt% total salt) of the mother liquor.
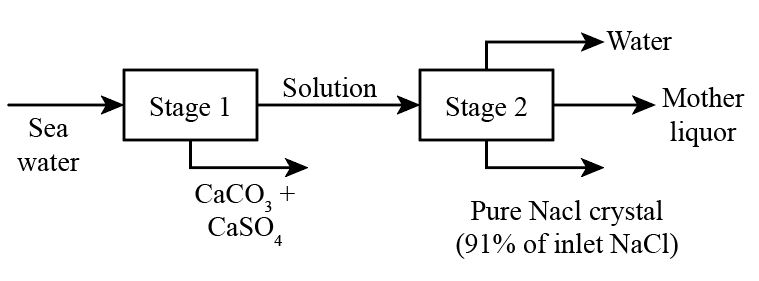
According to the question, the process can be shown by the following flow sheet
if we see both stages as a single unit
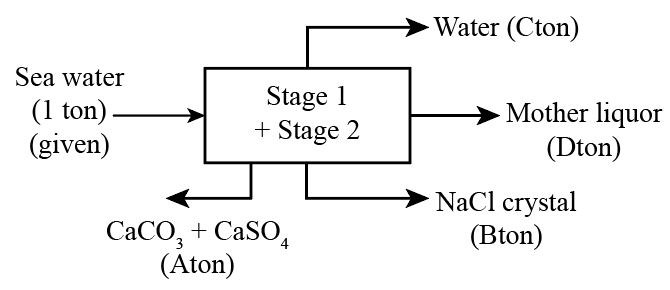
Let A,B,C,D be the mass flow rate of (CaCO3+CaSO4), NaCl, Water and mother liquor respectively
As shown in the Diagram, outle pure NaCl crystals contain 91% of the inlet NaCl
water in inlet sea water = water evaporated
thus water evaporated = 0.963 ton
By total material balance,
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 2 images









