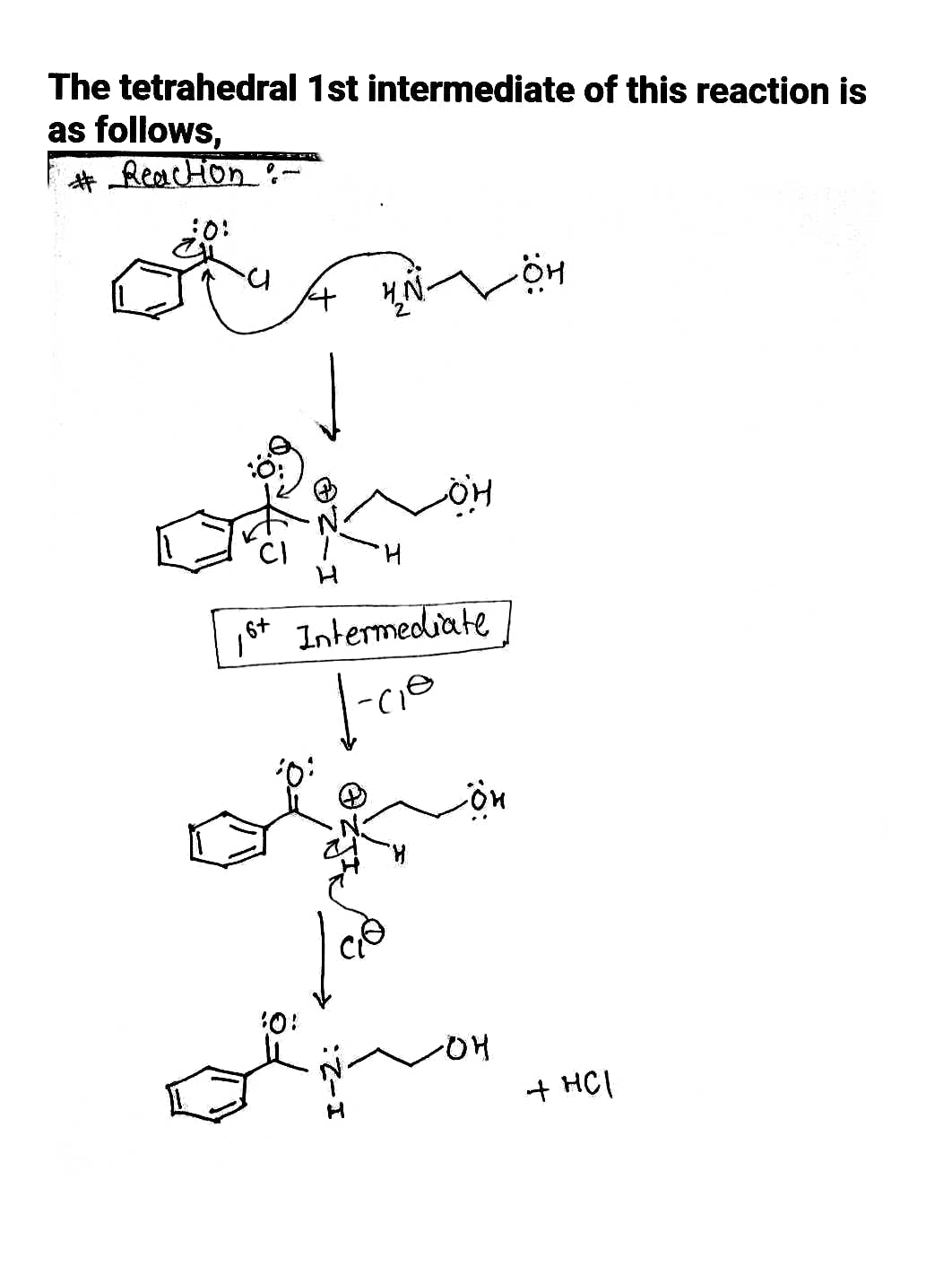
[Review Topics] [References] Acyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed the following reaction. + H₂N • You do not have to consider stereochemistry. . Include all valence lone pairs in your answer. OH Do not include counter-ions, e.g., Nat, I, in your answer. - In cases where there is more than one answer, just draw one.
[Review Topics] [References] Acyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed the following reaction. + H₂N • You do not have to consider stereochemistry. . Include all valence lone pairs in your answer. OH Do not include counter-ions, e.g., Nat, I, in your answer. - In cases where there is more than one answer, just draw one.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Draw the tetrahedral intermediate as it is first formed in the following reaction. I keep getting it wrong.

Transcribed Image Text:### Educational Content on Acyl Transfer Reactions
**Acyl transfer (nucleophilic substitution at carbonyl) reactions** proceed in two stages via a "tetrahedral intermediate." The task is to draw the tetrahedral intermediate **as it is first formed** in the following reaction:
**Reaction Components:**
- **Reactants:**
- An acyl chloride with a benzene ring (phenyl group) attached to a carbonyl group (C=O) and a chlorine atom.
- A compound containing an amine group (NH₂) and an alcohol group (OH).
- **Reaction Arrow:** Indicates the progression from reactants to products through the formation of an intermediate.
**Instructions:**
- **Stereochemistry:**
- You do not have to consider stereochemistry for this task.
- **Valence Lone Pairs:**
- Include all valence lone pairs in your drawing of the tetrahedral intermediate.
- **Counter-Ions:**
- Do not include counter-ions such as Na⁺ or I⁻ in your answer.
- **Multiple Possible Answers:**
- If there is more than one possible answer for the intermediate, draw only one.
**Diagrams and Tools:**
- Below the instruction text, various tool icons are visible which may be used for drawing chemical structures, indicating bonds, and incorporating lone pairs into diagrams.
### Note:
It is important to understand the mechanism of acyl transfer reactions and be able to illustrate intermediates, as they play a crucial role in organic synthesis. Accurate depiction of lone pairs and structural changes is essential for mastering reaction mechanisms.
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY