Regarding the following reaction at 298.15 K: 4 HCI (g) + O2(g) → 2Cl2 (g) + 2H₂O (1) The following table lists the stand enthalpy of formation, the standard entropy and the standard formation Gibbs energies
Regarding the following reaction at 298.15 K: 4 HCI (g) + O2(g) → 2Cl2 (g) + 2H₂O (1) The following table lists the stand enthalpy of formation, the standard entropy and the standard formation Gibbs energies
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
![### Question 2
**Regarding the following reaction at 298.15 K:**
\[ 4 \text{HCl (g)} + \text{O}_2 \text{(g)} \rightarrow 2 \text{Cl}_2 \text{(g)} + 2 \text{H}_2\text{O (l)} \]
The following table lists the standard enthalpy of formation, the standard entropy, and the standard formation Gibbs energies:
| | HCl (g) | O<sub>2</sub> (g) | Cl<sub>2</sub> (g) | H<sub>2</sub>O (l) |
|----------------|----------|------------------|-------------------|-------------------|
| ΔH<sub>f</sub>° (kJ/mol) | -92.31 | 0 | 0 | -285.83 |
| S° (J/K·mol) | 186.91 | 205.14 | 223.07 | 69.91 |
| ΔG<sub>f</sub>° (kJ/mol) | -95.3 | 0 | 0 | ? |
Please calculate the standard reaction entropy Δ<sub>r</sub>S° (J·K<sup>-1</sup>·mol<sup>-1</sup>), the standard reaction enthalpy Δ<sub>r</sub>H° (kJ·mol<sup>-1</sup>), the standard reaction Gibbs energy Δ<sub>r</sub>G° (kJ·mol<sup>-1</sup>), and the maximum nonexpansion work that can be gained from the oxidation of HCl (g) at 298.15 K.
**Instruction:** Please enter all answers with **two decimal places**, for example: -344.101 is written as -344.10. Pay attention to the units, particularly Δ<sub>r</sub>S° in J/(K·mol) while others are in kJ/mol.
1. Δ<sub>r</sub>S° (J·K<sup>-1</sup>·mol<sup>-1</sup>) = [_______]
2. Δ<sub>r</sub>H° (kJ·mol<sup>-](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ff4dfc165-1571-419f-99fc-a4587d76c340%2Fafabc16b-84a2-46d2-8dd2-9300496b318d%2Fnc97v4_processed.jpeg&w=3840&q=75)
Transcribed Image Text:### Question 2
**Regarding the following reaction at 298.15 K:**
\[ 4 \text{HCl (g)} + \text{O}_2 \text{(g)} \rightarrow 2 \text{Cl}_2 \text{(g)} + 2 \text{H}_2\text{O (l)} \]
The following table lists the standard enthalpy of formation, the standard entropy, and the standard formation Gibbs energies:
| | HCl (g) | O<sub>2</sub> (g) | Cl<sub>2</sub> (g) | H<sub>2</sub>O (l) |
|----------------|----------|------------------|-------------------|-------------------|
| ΔH<sub>f</sub>° (kJ/mol) | -92.31 | 0 | 0 | -285.83 |
| S° (J/K·mol) | 186.91 | 205.14 | 223.07 | 69.91 |
| ΔG<sub>f</sub>° (kJ/mol) | -95.3 | 0 | 0 | ? |
Please calculate the standard reaction entropy Δ<sub>r</sub>S° (J·K<sup>-1</sup>·mol<sup>-1</sup>), the standard reaction enthalpy Δ<sub>r</sub>H° (kJ·mol<sup>-1</sup>), the standard reaction Gibbs energy Δ<sub>r</sub>G° (kJ·mol<sup>-1</sup>), and the maximum nonexpansion work that can be gained from the oxidation of HCl (g) at 298.15 K.
**Instruction:** Please enter all answers with **two decimal places**, for example: -344.101 is written as -344.10. Pay attention to the units, particularly Δ<sub>r</sub>S° in J/(K·mol) while others are in kJ/mol.
1. Δ<sub>r</sub>S° (J·K<sup>-1</sup>·mol<sup>-1</sup>) = [_______]
2. Δ<sub>r</sub>H° (kJ·mol<sup>-

Transcribed Image Text:### Thermodynamics Exercise
1. **Change in Standard Entropy**
\(\Delta_rS^\circ (\text{JK}^{-1}\text{mol}^{-1}) = \)
2. **Change in Standard Enthalpy**
\(\Delta_rH^\circ (\text{kJmol}^{-1}) = \)
3. **Calculate the Standard Reaction Gibbs Energy**
Please use your results from (1) and (2) to calculate the standard reaction Gibbs energy:
\(\Delta_rG^\circ (\text{kJmol}^{-1}) = \)
4. **Gibbs Energy of Formation for H\(_2\)O (l)**
Use the result from (3) and the data below to calculate the standard formation Gibbs energy of H\(_2\)O (l):
\(\Delta_fG^\circ (\text{kJmol}^{-1}) = \)
5. **Maximum Work from Oxidation of HCl (g)**
\( w^{\text{max}} (\text{kJg}^{-1}) \) that can be gained from the oxidation of HCl (g) on a per gram basis = \(\_\_\_\_\_\)
*Note: The reaction equation contains 4 moles of HCl*
### Instructions
- Fill in the blanks with appropriate calculations.
- Use thermodynamic equations as needed to compute values.
- Ensure consistency in units across all calculations.
Expert Solution
The standard molar property change for a chemical reaction
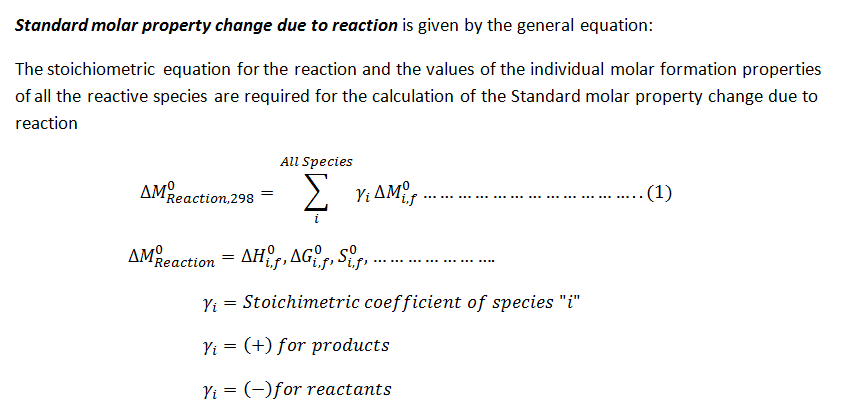
Step by step
Solved in 5 steps with 18 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The