Mellunal, CH3OH, foeerly kiwIs wIni siksshael, is nmulusturel menially by th: folkwing relim Col0) – 22(g) - CII,OH(g) A1S00L vessel was illed with 0.1s10 mel CO and 0.3910 mol Hy. Wben this mixce came to equilibrim at S00 K, the vessel conisined U.1280 el C0. Llow msuy males of each substane wene in the vessel al eguilibim? пю На mo. CH,OH
Mellunal, CH3OH, foeerly kiwIs wIni siksshael, is nmulusturel menially by th: folkwing relim Col0) – 22(g) - CII,OH(g) A1S00L vessel was illed with 0.1s10 mel CO and 0.3910 mol Hy. Wben this mixce came to equilibrim at S00 K, the vessel conisined U.1280 el C0. Llow msuy males of each substane wene in the vessel al eguilibim? пю На mo. CH,OH
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter12: Gaseous Chemical Equilibrium
Section: Chapter Questions
Problem 36QAP: At a certain temperature, K=0.29 for the decomposition of two moles of iodine trichloride, ICl3(s),...
Related questions
Question

Transcribed Image Text:Mellunal, CH3OH, foeerly kiwIs wIni siksshael, is nmulusturel menially by th: folkwing relim
Col0) – 22(g) - CII,OH(g)
A1S00L vessel was illed with 0.1s10 mel CO and 0.3910 mol Hy. Wben this mixce came to equilibrim at S00 K, the vessel conisined U.1280 el C0. Llow msuy males of each substane
wene in the vessel al eguilibim?
пю На
mo. CH,OH
Expert Solution
Step 1
Given:
Initial mole of CO = 0.1510 mol
Initial mole of H2 = 0.3910 mol
Moles of CO at equilibrium = 0.128 mol
Step 2
Determination of moles at equilibrium:
Construct ICE table.
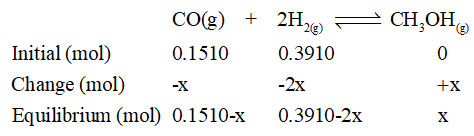
The given equilibrium moles of CO = 0.128 mol
Substitute this in equilibrium mole of CO from ICE table and solve for x.
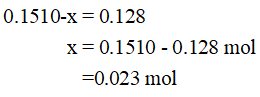
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax