II 8TIIL! US ng TaUie 2.9 UI page II II your tCxID0OK, IUchtIly tne 3U03UICC. USIlng 245a l,20 7Lx TNG= 1,2076x 1.20 7x lo ml -3 Txios= lx103 l.207yl0% 0.00201982€ Dimensional Analysis Applications Solve the following problems using dimensional analysis, where appropriate. Round to the correct number of significant figures and include units. Show all of your work. Please circle your final answer. 7. Methyl mercury levels higher than 3.00 × 104 mg/dL are toxic to the central nervous system. Determine if either of the following levels of methyl mercury is life-threatening. a) 2.50 µg/L b) 2.50 cg/mL Handbook 3 ©2020 Cuesta College. All rights reserved 15
II 8TIIL! US ng TaUie 2.9 UI page II II your tCxID0OK, IUchtIly tne 3U03UICC. USIlng 245a l,20 7Lx TNG= 1,2076x 1.20 7x lo ml -3 Txios= lx103 l.207yl0% 0.00201982€ Dimensional Analysis Applications Solve the following problems using dimensional analysis, where appropriate. Round to the correct number of significant figures and include units. Show all of your work. Please circle your final answer. 7. Methyl mercury levels higher than 3.00 × 104 mg/dL are toxic to the central nervous system. Determine if either of the following levels of methyl mercury is life-threatening. a) 2.50 µg/L b) 2.50 cg/mL Handbook 3 ©2020 Cuesta College. All rights reserved 15
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:II 8TIIL! US ng TaUie 2.9 UI page II II your tCxID0OK, IUchtIly tne 3U03UICC.
USIlng
245a
l,20 7Lx TNG=
1,2076x
1.20 7x lo ml
-3
Txios=
lx103
l.207yl0%
0.00201982€
Dimensional Analysis Applications
Solve the following problems using dimensional analysis, where appropriate. Round to the correct
number of significant figures and include units. Show all of your work. Please circle your final answer.
7. Methyl mercury levels higher than 3.00 × 104 mg/dL are toxic to the central nervous system.
Determine if either of the following levels of methyl mercury is life-threatening.
a) 2.50 µg/L
b) 2.50 cg/mL
Handbook 3
©2020 Cuesta College. All rights reserved
15
Expert Solution
Step 1
Methyl mercury levels higher than 3.00×10-4 mg/dL are toxic to central nervous system. Whether the given levels of methyl mercury are life threatening or not is to be determined-
1. 50 µg/L
- 50 cg/mL
Step 2
- Convert the unit µg/L into mg/dL
1 µg = 0.001 mg
1 L = 10 dL (decilitres)
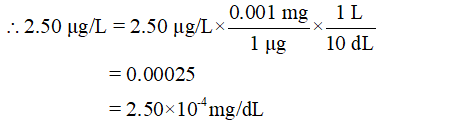
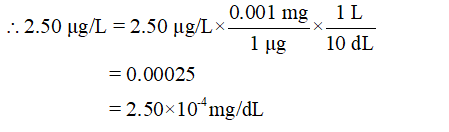
Step 3
Thus, 2.50×10-4 mg/dL < 3.00×10-4 mg/dL.
Hence, 2.50 µg/L levels of methyl mercury is not life threatening.
Step by step
Solved in 5 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY