PQ PQ WSJ ← → CO app.101edu.co M Inbox (38) - armaan... CAS Login - CAS-Centr... STARTING AMOUNT X YouTube g Na Chemi X lc Dashbox 65.02 ADD FACTOR x( ) Oracle PeopleSoft S... HC Highline College D... Hc Dates and Deadline... 1 77.7 g N₂/L What mass in grams of NaNs is required to produce 50.2 L of N₂ gas (density = 1.25 g/L) according to the balanced chemical reaction: Logout X 6.022 x 10²3 2 Question 40 of 40 mol NaNs 2 NaN:(s)→→ 2 Na(s) + 3 N2(g) Cenga x 3 g N₂ 146 ANSWER 1.25 mol N₂ Aktiv X 22.99 97.1 g NaNs 28.02 B [Solve X Microsoft Office Ho... Highline College Ru... RESET 5 50.2 LN₂ 218 Q how to X + mol Na ★ 0 llc Associate Degree &... A ⠀ + >>> Submit
PQ PQ WSJ ← → CO app.101edu.co M Inbox (38) - armaan... CAS Login - CAS-Centr... STARTING AMOUNT X YouTube g Na Chemi X lc Dashbox 65.02 ADD FACTOR x( ) Oracle PeopleSoft S... HC Highline College D... Hc Dates and Deadline... 1 77.7 g N₂/L What mass in grams of NaNs is required to produce 50.2 L of N₂ gas (density = 1.25 g/L) according to the balanced chemical reaction: Logout X 6.022 x 10²3 2 Question 40 of 40 mol NaNs 2 NaN:(s)→→ 2 Na(s) + 3 N2(g) Cenga x 3 g N₂ 146 ANSWER 1.25 mol N₂ Aktiv X 22.99 97.1 g NaNs 28.02 B [Solve X Microsoft Office Ho... Highline College Ru... RESET 5 50.2 LN₂ 218 Q how to X + mol Na ★ 0 llc Associate Degree &... A ⠀ + >>> Submit
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
I need help on this question using the conversion analysis
![**Question 40 of 40**
What mass in grams of NaN₃ is required to produce 50.2 L of N₂ gas (density = 1.25 g/L) according to the balanced chemical reaction:
\[ 2 \, \text{NaN}_3(s) \rightarrow 2 \, \text{Na}(s) + 3 \, \text{N}_2(g) \]
**Diagram Explanation:**
1. **Starting Amount Section:**
- Two empty input boxes represent the initial quantities of reactants or products, labeled as "STARTING AMOUNT."
2. **Calculation Section:**
- A multiplication setup with input fields enclosed in parentheses for calculating molar masses or stoichiometry.
- An “ANSWER” box for displaying the calculated result.
- A “RESET” button to clear inputs and start over.
3. **Add Factor Section:**
- Provides numeric buttons, such as molar masses and volume, including:
- 65.02, 77.7, 6.022 x 10²³ (likely referring to molar masses or Avogadro's number).
- Other factors like 1, 2, 50.2, etc.
- Buttons labeled with units like “g Na,” “g NaN₃,” “mol NaN₃,” and others for stoichiometric calculations.
This exercise involves interpreting a balanced chemical equation and using given values to determine the required mass of a reactant to produce a specific volume of a gaseous product.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fcb6887dc-5b49-4551-bc80-996c7c4ae414%2F30446a8f-658f-454d-9f45-47ff38af06a5%2Fvpkoc72i_processed.png&w=3840&q=75)
Transcribed Image Text:**Question 40 of 40**
What mass in grams of NaN₃ is required to produce 50.2 L of N₂ gas (density = 1.25 g/L) according to the balanced chemical reaction:
\[ 2 \, \text{NaN}_3(s) \rightarrow 2 \, \text{Na}(s) + 3 \, \text{N}_2(g) \]
**Diagram Explanation:**
1. **Starting Amount Section:**
- Two empty input boxes represent the initial quantities of reactants or products, labeled as "STARTING AMOUNT."
2. **Calculation Section:**
- A multiplication setup with input fields enclosed in parentheses for calculating molar masses or stoichiometry.
- An “ANSWER” box for displaying the calculated result.
- A “RESET” button to clear inputs and start over.
3. **Add Factor Section:**
- Provides numeric buttons, such as molar masses and volume, including:
- 65.02, 77.7, 6.022 x 10²³ (likely referring to molar masses or Avogadro's number).
- Other factors like 1, 2, 50.2, etc.
- Buttons labeled with units like “g Na,” “g NaN₃,” “mol NaN₃,” and others for stoichiometric calculations.
This exercise involves interpreting a balanced chemical equation and using given values to determine the required mass of a reactant to produce a specific volume of a gaseous product.
Expert Solution
Step 1
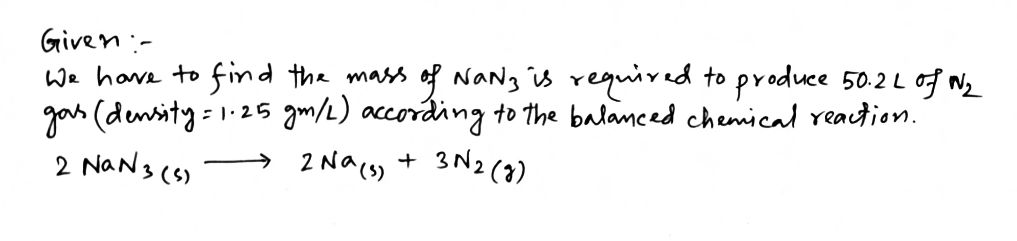
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY