How many grams of KNO, (101.10g mol ") must be added to 102 g water to produce a boiling point elevation equl to the magnitude of a freezing point depression observed for a solution of 203 g of MgC, (95 21 g mol )in 191gwate. assuming complete dissociation? Kb= 0.52°C m, Kf = 1.86 °C m* for water 0 1211g 11.57g 0 3.61g 6.18g 15.28g dudur Gdle

Each solution possesses particular boiling point and also freezing point. The increment/elevation in any solution's boiling point after adding any specified solute is considered as "boiling point elevation". Whereas the decrement in any solution's freezing point is "freezing point depression".

The calculation of freezing point depression for MgCl2
The number of moles of MgCl2 can be calculated as shown below.
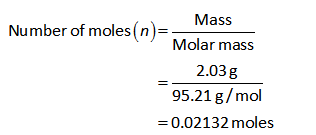
The number of moles of MgCl2 is 0.02132 moles.
The molality of the MgCl2 solution can be calculated as shown below.
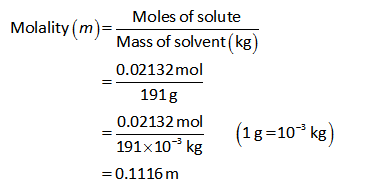
Thus the molality of MgCl2 solution is 0.1116 m.
The formula for the calculation of freezing point depression is shown below.

Since MgCl2 is an electrolyte and in aqueous solution, 1 mole of MgCl2 dissociates into 1 mole of Mg2+ and 2 moles of Cl- ions.
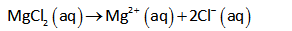
Thus the Van’t Hoff factor (i) for MgCl2 in water is 3.

The calculation of boiling point elevation for KNO3
The formula for the calculation of boiling point elevation is shown below.


Thus the Van’t Hoff factor (i) for KNO3 in water is 2.
Step by step
Solved in 7 steps with 12 images









