Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
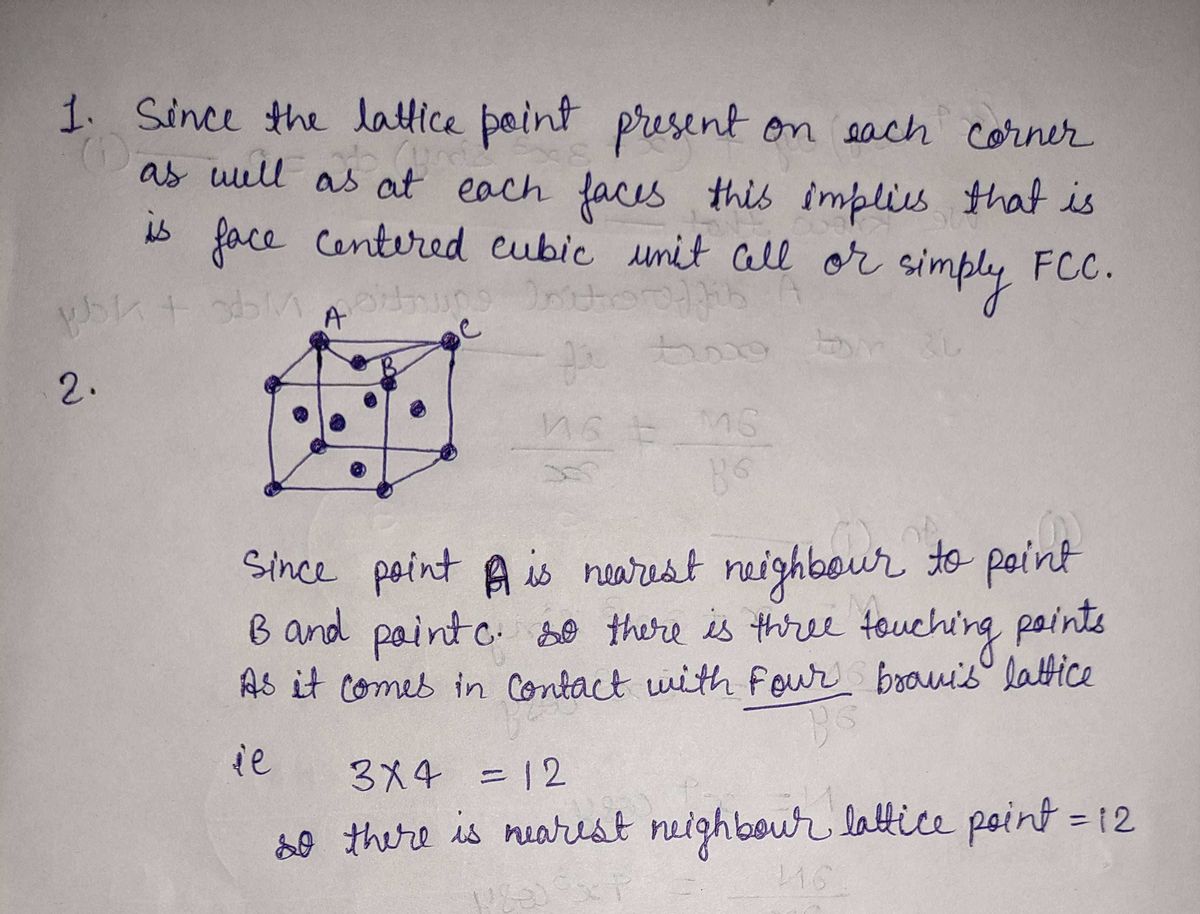
Given the following Bravais lattices:
1- Identify (describe) the Bravais lattice
2- How many nearest neighbor lattice points are there for each lattice point in the lattice four lattice type?
3- How many lattices points are in each unit cell?
4- Determine the shortest distance between particles in each unit cell.

Transcribed Image Text:This image illustrates a crystal lattice structure, specifically representing a Body-Centered Cubic (BCC) lattice.
### Description of the Structure:
- **Atoms Representation**: The lattice consists of spheres that depict atoms. There are two distinct types of spheres:
- **Green Spheres**: These are positioned at the corners of the cube as well as at the center, indicating the primary lattice points where the atoms are located.
- **White Spheres**: These represent additional atoms located at the center of the cube, typical of a BCC structure.
- **Connections**: Lines connect the spheres, symbolizing the chemical bonds or interactions between these atoms at the lattice points.
### Explanation of the BCC Lattice:
The Body-Centered Cubic (BCC) structure is characterized by having one atom at each corner of the cube and one atom in the very center of the cube. This arrangement contributes to its high packing efficiency and is commonly found in metals such as iron, chromium, and tungsten.
### Key Features:
- **Coordination Number**: The BCC structure has a coordination number of 8, meaning each central atom is directly connected to 8 other atoms.
- **Atomic Packing Factor**: The packing efficiency for BCC is approximately 68%.
This representation is crucial for understanding the physical and chemical properties of materials that crystallize in a BCC structure, affecting their density, melting point, and mechanical properties.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY