b) The standard enthalpy change of combustion (i.e. the heat change) of sucrose is -5644 kJ mol-¹. Calculate the energy released when one sweet containing 6.70 g of sucrose is completely burnt. c) A man needs to consume about 2500 dietary calories (i.e. 2500 kcal) per day. Given that 1 kJ = 0.239 kcal, how many sweets would a man have to consume in order to meet his daily calorific requirement?
b) The standard enthalpy change of combustion (i.e. the heat change) of sucrose is -5644 kJ mol-¹. Calculate the energy released when one sweet containing 6.70 g of sucrose is completely burnt. c) A man needs to consume about 2500 dietary calories (i.e. 2500 kcal) per day. Given that 1 kJ = 0.239 kcal, how many sweets would a man have to consume in order to meet his daily calorific requirement?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:b) The standard enthalpy change of combustion (i.e. the heat change) of sucrose is -5644 kJ
mol-¹. Calculate the energy released when one sweet containing 6.70 g of sucrose is completely
burnt.
c) A man needs to consume about 2500 dietary calories (i.e. 2500 kcal) per day. Given that 1 kJ
= 0.239 kcal, how many sweets would a man have to consume in order to meet his daily
calorific requirement?
Sherbet produces a fizzing sensation in the mouth when its two main components, tartaric acid
and sodium hydrogencarbonate, react together in aqueous solution to make carbon dioxide gas.
The reaction is given below:
H₂C₂H4O6 + 2NaHCO3
Sodium
Bicarbonate
Tartaric Acid
Na₂C4H₂O6 + 2H₂O +
Sodium
Water
Tartarate
2CO₂
Carbon
Dioxide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
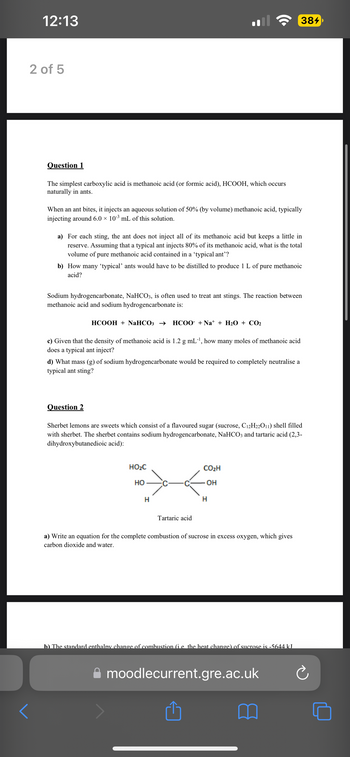
Transcribed Image Text:12:13
2 of 5
D
<
Question 1
The simplest carboxylic acid is methanoic acid (or formic acid), HCOOH, which occurs
naturally in ants.
When an ant bites, it injects an aqueous solution of 50% (by volume) methanoic acid, typically
injecting around 6.0 × 10-³ mL of this solution.
a) For each sting, the ant does not inject all of its methanoic acid but keeps a little in
reserve. Assuming that a typical ant injects 80% of its methanoic acid, what is the total
volume of pure methanoic acid contained in a 'typical ant'?
b) How many 'typical' ants would have to be distilled to produce 1 L of pure methanoic
acid?
Sodium hydrogencarbonate, NaHCO3, is often used to treat ant stings. The reaction between
methanoic acid and sodium hydrogencarbonate is:
HCOOH + NaHCO3 → HCOO +Na* + H2O + CO2
c) Given that the density of methanoic acid is 1.2 g mL-¹, how many moles of methanoic acid
does a typical ant inject?
d) What mass (g) of sodium hydrogencarbonate would be required to completely neutralise a
typical ant sting?
Question 2
Sherbet lemons are sweets which consist of a flavoured sugar (sucrose, C12H22O11) shell filled
with sherbet. The sherbet contains sodium hydrogencarbonate, NaHCO3 and tartaric acid (2,3-
dihydroxybutanedioic acid):
HO₂C
HO
H
Tartaric acid
384
CO₂H
OH
H
a) Write an equation for the complete combustion of sucrose in excess oxygen, which gives
carbon dioxide and water.
b) The standard enthalpy change of combustion (i.e. the heat change) of sucrose is -5644 kJ
moodlecurrent.gre.ac.uk
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY