A radio station's channel, such as 100.7 FM or 92.3 FM, is actually its frequency in megahertz (MHz), where 1 MHz = 10° Hz and 1 Hz = 1 s Calculate the broadcast wavelength of the radio station 107.9 FM. Express your answer to four significant figures and include the appropriate units. • View Available Hint(s) HA ?
A radio station's channel, such as 100.7 FM or 92.3 FM, is actually its frequency in megahertz (MHz), where 1 MHz = 10° Hz and 1 Hz = 1 s Calculate the broadcast wavelength of the radio station 107.9 FM. Express your answer to four significant figures and include the appropriate units. • View Available Hint(s) HA ?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:Learning Goal:
Several properties are used to define waves. Every wave has a
wavelength, which is the distance from peak to peak or trough to
trough. Wavelength, typically given the symbol A (lowercase Greek
"lambda"), is usually measured in meters. Every wave also has a
frequency, which is the number of wavelengths that pass a certain
point during a given period of time. Frequency, given the symbol v
(lowercase Greek "nu"), is usually measured in inverse seconds
(s). Hertz (Hz), another unit of frequency, is equivalent to inverse
seconds.
The product of wavelength and frequency is the speed in meters
per second (m/s). For light waves, the speed is constant. The
speed of light is symbolized by the letter c and is always equal to
2.998 x 10° m/s in a vacuum, that is,
c = \v = 2.998 x 10° m/s
Another term for "light" is electromagnetic radiation, which
encompasses not only visible light but also gamma rays, X-rays, UV
rays, infrared rays, microwaves, and radio waves. As you could
probably guess, these different kinds of radiation are associated
with different energy regimes. Gamma rays have the greatest
energy, whereas radio waves have the least energy. The energy
(measured in joules) of a photon for a particular kind of light wave is
equal to its frequency times a constant called Planck's constant,
symbolized h :
Ephoton = hv
where
h 6.626 x 10 34 J.s
These two equations can be combined to give an equation that
relates energy to wavelength:
E
hc

Transcribed Image Text:Part A
A radio station's channel, such as 100.7 FM or 92.3 EM, is actually its frequency in megahertz (MHz), where 1 MHz = 10° Hz and 1 Hz = 1s1
Calculate the broadcast wavelength of the radio station 107.9 FM.
Express your answer to four significant figures and include the appropriate units.
> View Available Hint(s)
µA
?
Value
Units
Submit
Previous Answers
X Incorrect; Try Again; 2 attempts remaining
Expert Solution
Step 1
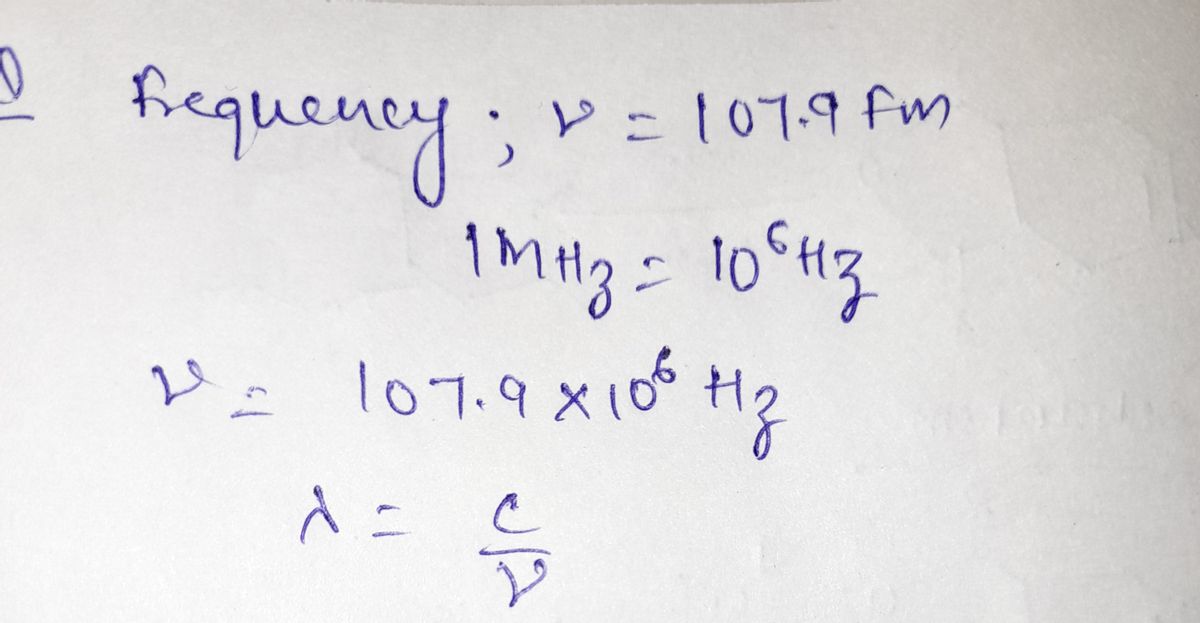
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY